Preventive migraine treatment with eptinezumab reduced acute headache medication and headache frequency to below diagnostic thresholds in patients with chronic migraine and medication-overuse headache
- PMID: 34551130
- PMCID: PMC9291601
- DOI: 10.1111/head.14206
Preventive migraine treatment with eptinezumab reduced acute headache medication and headache frequency to below diagnostic thresholds in patients with chronic migraine and medication-overuse headache
Abstract
Objective: This post hoc analysis in patients medically diagnosed with chronic migraine (CM) and medication-overuse headache (MOH) evaluated reductions in the use of acute headache medication (AHM) and sustained changes in the diagnostic status of CM and MOH following eptinezumab treatment in the PROMISE-2 study.
Background: Eptinezumab, a monoclonal antibody that inhibits calcitonin gene-related peptide, is approved in the United States for the preventive treatment of migraine. A previous analysis showed that eptinezumab reduced monthly migraine days and was well tolerated in the subgroup of PROMISE-2 patients diagnosed with both CM and MOH.
Methods: The phase 3, double-blind, placebo-controlled PROMISE-2 study (NCT02974153) randomized adults with CM to eptinezumab 100 mg, 300 mg, or placebo (administered intravenously every 12 weeks for up to two doses). MOH was prospectively diagnosed at screening by trained physicians based on 3 months of medication history and International Classification of Headache Disorders-3β criteria. This post hoc analysis evaluated changes in total and class-specific days of AHM usage, the percentage of patients using AHM at or above MOH diagnostic thresholds, and the percentage of patients experiencing monthly headache and migraine day frequency below diagnostic thresholds for MOH and/or CM.
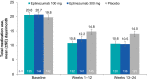
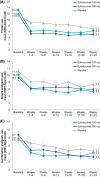
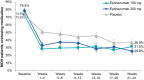
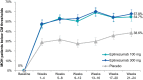
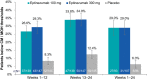
Results: In PROMISE-2, 431/1072 (40.2%) patients with CM were diagnosed with MOH (eptinezumab 100 mg, n = 139; 300 mg, n = 147; placebo, n = 145) and were included in this analysis. Total monthly AHM use decreased from 20.6 days/month at baseline to 10.6 days/month over 24 weeks of treatment (49% decrease) with eptinezumab 100 mg, from 20.7 to 10.5 days/month (49% decrease) with eptinezumab 300 mg, and from 19.8 to 14.0 days/month (29% decrease) with placebo. Numerically greater decreases from baseline with eptinezumab were also observed for individual drug classes. In each study month, the percentages of patients who were below MOH thresholds were numerically higher for both eptinezumab doses compared with placebo, as were the percentages of patients experiencing headache and migraine frequency below CM thresholds. Of patients with available data across the entire treatment period, 29.0% (58/200) of patients treated with eptinezumab stopped meeting and remained below diagnostic thresholds for both CM and MOH during Weeks 1-24, as well as 6.3% (6/96) of patients who received placebo.
Conclusions: Across 24 weeks of treatment, eptinezumab reduced AHM use in patients diagnosed with CM and MOH. More than one-fourth (29%) of patients treated with eptinezumab did not meet the diagnostic thresholds for either CM or MOH for the entire treatment period.
Keywords: acute headache medication use; chronic migraine; eptinezumab; medication-overuse headache.
© 2021 The Authors. Headache: The Journal of Head and Face Pain published by Wiley Periodicals LLC on behalf of American Headache Society.
Conflict of interest statement
M. J. Marmura: Grants for research (no personal compensation): Allergan/Abbvie, ElectroCore, and Teva; Consultant and/or advisory board member: Alder/Lundbeck, Amgen/Novartis, Antres, Promius, Supernus, Theranica, and Valeant; Speaker's bureau: Amgen/Novartis, and Eli Lilly; Royalties: Cambridge, Demos Medical, and MedLink. H.‐C. Diener: Honoraria for participation in clinical trials, contribution to advisory boards, or oral presentations over the last 3 years: Alder, Allergan, Amgen, Bristol‐Myers Squibb, Electrocore, Ipsen Pharma, Lundbeck, Lilly, MSD, Novartis, Pfizer, Teva, and Weber & Weber; Financial research support: Allergan and Electrocore; Headache research support: German Research Council (DFG), the German Ministry of Education and Research (BMBF), and the European Union; Editorial boards:
Figures
References
-
- Diener HC, Dodick D, Evers S, et al. Pathophysiology, prevention, and treatment of medication overuse headache. Lancet Neurol. 2019;18:891‐902. - PubMed
-
- Headache Classification Committee of the International Headache Society (IHS) . The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38:1‐211. - PubMed
-
- Linde M, Gustavsson A, Stovner LJ, et al. The cost of headache disorders in Europe: the Eurolight project. Eur J Neurol. 2012;19:703‐711. - PubMed
-
- Bendtsen L, Munksgaard S, Tassorelli C, et al. Disability, anxiety and depression associated with medication‐overuse headache can be considerably reduced by detoxification and prophylactic treatment. Results from a multicentre, multinational study (COMOESTAS project). Cephalalgia. 2014;34:426‐433. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

