Negative immune responses to two-dose mRNA COVID-19 vaccines in renal allograft recipients assessed with simple antibody and interferon gamma release assay cellular monitoring
- PMID: 34551187
- PMCID: PMC8653097
- DOI: 10.1111/ajt.16854
Negative immune responses to two-dose mRNA COVID-19 vaccines in renal allograft recipients assessed with simple antibody and interferon gamma release assay cellular monitoring
Abstract
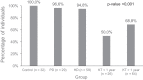
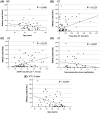
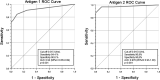
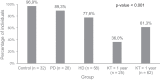
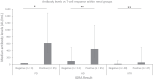
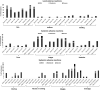
Studies are urgently needed to characterize immunogenicity, efficacy, and safety of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) mRNA vaccines in kidney transplant (KT) recipients, excluded from major clinical trials. Complex ELISPOT and other cellular response techniques have been applied, but simpler tools are needed. An easy-to-use real-world monitoring of SARS-CoV-2 IgG antibodies against the Spike protein and QuantiFERON® SARS-CoV-2 IFNγ release assay (IGRA) were performed at baseline and 28 days after the second dose in KT recipients and controls (dialysis patients and healthy ones). All healthy controls and >95% dialysis controls became positive for anti-S IgG antibodies, while only 63.3% of KT patients seroconverted with a very low antibody level. A positive IGRA was documented in 96.9% of controls, 89.3% peritoneal dialysis, 77.6% hemodialysis, 61.3% of KT patients transplanted more than 1 year ago and only 36% of those transplanted within the previous 12 months. Overall, 100% of healthy controls, 95.4% of dialysis patients and 78.8% KT recipients developed any immune response (humoral and/or cellular) against SARS-CoV-2. KT patients showed low rates of immune responses to mRNA Coronavirus infectious disease 2019 vaccines, especially those with recent transplantations. Simple humoral and cellular monitoring is advisable, so that repeated doses may be scheduled according to the results.
Keywords: COVID-19; T cell biology; antibody biology; clinical research/practice; dialysis; immunobiology; infectious disease; kidney transplantation/nephrology; vaccine.
© 2021 The American Society of Transplantation and the American Society of Transplant Surgeons.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

