Effectiveness of mRNA Covid-19 Vaccine among U.S. Health Care Personnel
- PMID: 34551224
- PMCID: PMC8482809
- DOI: 10.1056/NEJMoa2106599
Effectiveness of mRNA Covid-19 Vaccine among U.S. Health Care Personnel
Abstract
Background: The prioritization of U.S. health care personnel for early receipt of messenger RNA (mRNA) vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (Covid-19), allowed for the evaluation of the effectiveness of these new vaccines in a real-world setting.
Methods: We conducted a test-negative case-control study involving health care personnel across 25 U.S. states. Cases were defined on the basis of a positive polymerase-chain-reaction (PCR) or antigen-based test for SARS-CoV-2 and at least one Covid-19-like symptom. Controls were defined on the basis of a negative PCR test for SARS-CoV-2, regardless of symptoms, and were matched to cases according to the week of the test date and site. Using conditional logistic regression with adjustment for age, race and ethnic group, underlying conditions, and exposures to persons with Covid-19, we estimated vaccine effectiveness for partial vaccination (assessed 14 days after receipt of the first dose through 6 days after receipt of the second dose) and complete vaccination (assessed ≥7 days after receipt of the second dose).
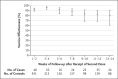
Results: The study included 1482 case participants and 3449 control participants. Vaccine effectiveness for partial vaccination was 77.6% (95% confidence interval [CI], 70.9 to 82.7) with the BNT162b2 vaccine (Pfizer-BioNTech) and 88.9% (95% CI, 78.7 to 94.2) with the mRNA-1273 vaccine (Moderna); for complete vaccination, vaccine effectiveness was 88.8% (95% CI, 84.6 to 91.8) and 96.3% (95% CI, 91.3 to 98.4), respectively. Vaccine effectiveness was similar in subgroups defined according to age (<50 years or ≥50 years), race and ethnic group, presence of underlying conditions, and level of patient contact. Estimates of vaccine effectiveness were lower during weeks 9 through 14 than during weeks 3 through 8 after receipt of the second dose, but confidence intervals overlapped widely.
Conclusions: The BNT162b2 and mRNA-1273 vaccines were highly effective under real-world conditions in preventing symptomatic Covid-19 in health care personnel, including those at risk for severe Covid-19 and those in racial and ethnic groups that have been disproportionately affected by the pandemic. (Funded by the Centers for Disease Control and Prevention.).
Copyright © 2021 Massachusetts Medical Society.
Figures
References
-
- Food and Drug Administration. Pfizer-BioNTech COVID-19 vaccine emergency use authorization. 2020. (https://www.fda.gov/emergency-preparedness-and-response/coronavirus-dise...).
-
- Food and Drug Administration. Moderna COVID-19 vaccine emergency use authorization. 2020. (https://www.fda.gov/emergency-preparedness-and-response/coronavirus-dise...).
-
- Food and Drug Administration. FDA approves first COVID-19 vaccine. 2021. (https://www.fda.gov/news-events/press-announcements/fda-approves-first-c...).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
