Efficacy of the mRNA-1273 SARS-CoV-2 Vaccine at Completion of Blinded Phase
- PMID: 34551225
- PMCID: PMC8482810
- DOI: 10.1056/NEJMoa2113017
Efficacy of the mRNA-1273 SARS-CoV-2 Vaccine at Completion of Blinded Phase
Abstract
Background: At interim analysis in a phase 3, observer-blinded, placebo-controlled clinical trial, the mRNA-1273 vaccine showed 94.1% efficacy in preventing coronavirus disease 2019 (Covid-19). After emergency use of the vaccine was authorized, the protocol was amended to include an open-label phase. Final analyses of efficacy and safety data from the blinded phase of the trial are reported.
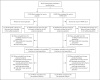
Methods: We enrolled volunteers who were at high risk for Covid-19 or its complications; participants were randomly assigned in a 1:1 ratio to receive two intramuscular injections of mRNA-1273 (100 μg) or placebo, 28 days apart, at 99 centers across the United States. The primary end point was prevention of Covid-19 illness with onset at least 14 days after the second injection in participants who had not previously been infected with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The data cutoff date was March 26, 2021.
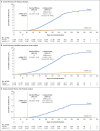
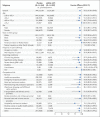
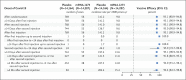
Results: The trial enrolled 30,415 participants; 15,209 were assigned to receive the mRNA-1273 vaccine, and 15,206 to receive placebo. More than 96% of participants received both injections, 2.3% had evidence of SARS-CoV-2 infection at baseline, and the median follow-up was 5.3 months in the blinded phase. Vaccine efficacy in preventing Covid-19 illness was 93.2% (95% confidence interval [CI], 91.0 to 94.8), with 55 confirmed cases in the mRNA-1273 group (9.6 per 1000 person-years; 95% CI, 7.2 to 12.5) and 744 in the placebo group (136.6 per 1000 person-years; 95% CI, 127.0 to 146.8). The efficacy in preventing severe disease was 98.2% (95% CI, 92.8 to 99.6), with 2 cases in the mRNA-1273 group and 106 in the placebo group, and the efficacy in preventing asymptomatic infection starting 14 days after the second injection was 63.0% (95% CI, 56.6 to 68.5), with 214 cases in the mRNA-1273 group and 498 in the placebo group. Vaccine efficacy was consistent across ethnic and racial groups, age groups, and participants with coexisting conditions. No safety concerns were identified.
Conclusions: The mRNA-1273 vaccine continued to be efficacious in preventing Covid-19 illness and severe disease at more than 5 months, with an acceptable safety profile, and protection against asymptomatic infection was observed. (Funded by the Biomedical Advanced Research and Development Authority and the National Institute of Allergy and Infectious Diseases; COVE ClinicalTrials.gov number, NCT04470427.).
Copyright © 2021 Massachusetts Medical Society.
Figures

References
-
- Johnson & Johnson COVID-19 vaccine authorized by U.S. FDA for emergency use — first single-shot vaccine in fight against global pandemic. New Brunswick, NJ: Johnson & Johnson, February 27, 2021. (https://www.jnj.com/johnson-johnson-covid-19-vaccine-authorized-by-u-s-f...).
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- UM1 AI148689/AI/NIAID NIH HHS/United States
- I01 BX003714/BX/BLRD VA/United States
- UM1 AI069424/AI/NIAID NIH HHS/United States
- UM1 AI148685/AI/NIAID NIH HHS/United States
- UM1 AI068618/AI/NIAID NIH HHS/United States
- UM1 AI148373/AI/NIAID NIH HHS/United States
- UM1 AI148684/AI/NIAID NIH HHS/United States
- L40 AI154610/AI/NIAID NIH HHS/United States
- UM1 AI068635/AI/NIAID NIH HHS/United States
- UM1 AI068619/AI/NIAID NIH HHS/United States
- K23 AI159399/AI/NIAID NIH HHS/United States
- UM1 AI068636/AI/NIAID NIH HHS/United States
- K08 AI143923/AI/NIAID NIH HHS/United States
- P30 ES010126/ES/NIEHS NIH HHS/United States
- UM1 AI148575/AI/NIAID NIH HHS/United States
- UM1 AI148576/AI/NIAID NIH HHS/United States
- UM1 AI068614/AI/NIAID NIH HHS/United States
- K12 HD052892/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
