Mitochondrial quality control in acute ischemic stroke
- PMID: 34551609
- PMCID: PMC8669286
- DOI: 10.1177/0271678X211046992
Mitochondrial quality control in acute ischemic stroke
Abstract
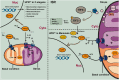
Mitochondria play a central role in the pathophysiological processes of acute ischemic stroke. Disruption of the cerebral blood flow during acute ischemic stroke interrupts oxygen and glucose delivery, leading to the dysfunction of mitochondrial oxidative phosphorylation and cellular bioenergetic stress. Cells can respond to such stress by activating mitochondrial quality control mechanisms, including the mitochondrial unfolded protein response, mitochondrial fission and fusion, mitophagy, mitochondrial biogenesis, and intercellular mitochondrial transfer. Collectively, these adaptive response strategies contribute to retaining the integrity and function of the mitochondrial network, thereby helping to recover the homeostasis of the neurovascular unit. In this review, we focus on mitochondrial quality control mechanisms occurring in acute ischemic stroke. A better understanding of how these regulatory pathways work in maintaining mitochondrial homeostasis will provide a rationale for developing innovative neuroprotectants when these mechanisms fail in acute ischemic stroke.
Keywords: Acute ischemic stroke; intercellular mitochondrial transfer; mitochondrial biogenesis; mitochondrial dynamics; mitochondrial quality control; mitochondrial unfolded protein response; mitophagy.
Conflict of interest statement
Figures


References
-
- Lees KR, Bluhmki E, von Kummer R, ECASS, ATLANTIS, NINDS and EPITHET rt-PA Study Group et al.. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet 2010; 375: 1695–1703. - PubMed
-
- Goyal M, Menon BK, van Zwam WH, et al.. Endovascular thrombectomy after large-vessel ischaemic stroke: a Meta-analysis of individual patient data from five randomised trials. Lancet 2016; 387: 1723–1731. - PubMed
-
- Nogueira RG, Jadhav AP, Haussen DC, et al.. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med 2018; 378: 11–21. - PubMed
-
- Savitz SI, Baron JC, Yenari MA, et al.. Reconsidering neuroprotection in the reperfusion era. Stroke 2017; 48: 3413–3419. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

