Transcriptional profiling reveals changes in gene regulation and signaling transduction pathways during temperature stress in wucai (Brassica campestris L.)
- PMID: 34551703
- PMCID: PMC8456696
- DOI: 10.1186/s12864-021-07981-9
Transcriptional profiling reveals changes in gene regulation and signaling transduction pathways during temperature stress in wucai (Brassica campestris L.)
Abstract
Background: Wucai (Brassica campestris L. ssp. chinensis var. rosularis Tsen) is a cold-tolerant plant that is vulnerable to high temperature. This study explored the response mechanism of wucai to low temperature. In this study, wucai seedlings were treated with different temperatures, including low temperature (LT), high temperature (HT), and a control.
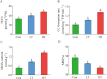
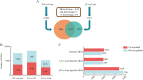
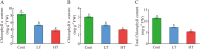
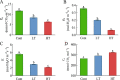
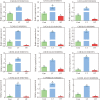
Results: According to transcriptomics analysis, the number of differentially expressed genes (DEGs) in HT and LT was 10,702 and 7267, respectively, compared with the control. The key genes associated with the physiological response of wucai to the treatments were analyzed. The Kyoto Encyclopedia of Genes and Genomes and Gene Ontology annotations indicated the importance of the photosynthesis and photosynthetic-antenna protein pathways. We found that a high-temperature environment greatly inhibited the expression of important genes in the photosynthetic pathway (BrLhc superfamily members, PsaD, PsaE, PsaD, PsaD, PsbO, PsbP, PsbQ, PsbR, PsbS, PsbW, PsbY, Psb27, and Psb28), whereas low temperature resulted in the expression of certain key genes (BrLhc superfamily members, Psa F, Psa H, Psb S, Psb H, Psb 28). In addition, the wucai seedlings exhibited better photosynthetic performance under low-temperature conditions than high-temperature conditions.
Conclusions: Based on the above results, we speculate that upon exposure to low temperature, the plants developed higher cold tolerance by upregulating the expression of genes related to photosynthesis. Conversely, high-temperature stress inhibited the expression of pivotal genes and weakened the self-regulating ability of the plants.
Keywords: BrLhc superfamily; Differentially expressed genes; Photosynthesis; RNA-Seq; Temperature stress; Wucai.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
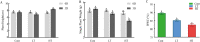





References
-
- Wahid A, Gelani S, Ashraf M, Foolad MR. Heat tolerance in plants: an overview. Environ Exp Bot. 2007;61(3):199–223. doi: 10.1016/j.envexpbot.2007.05.011. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

