Upconverting phosphor technology-based lateral flow assay for the rapid and sensitive detection of anti-Trichinella spiralis IgG antibodies in pig serum
- PMID: 34551787
- PMCID: PMC8456594
- DOI: 10.1186/s13071-021-04949-2
Upconverting phosphor technology-based lateral flow assay for the rapid and sensitive detection of anti-Trichinella spiralis IgG antibodies in pig serum
Abstract
Background: Trichinella spiralis is a zoonotic food-borne parasite. A disease caused by infection with T. spiralis is called trichinellosis in humans. It is important to investigate the epidemic situation and the surveillance of herds and then prevent infection in humans. Therefore, this study is to develop a rapid and sensitive diagnostic method for on-site test in domestic and wild animals.
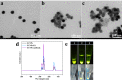
Methods: Upconverting phosphor nanoparticles (UCNPs), an excellent optical label, were conjugated with the excretory-secretory (ES) antigens from T. spiralis muscle larvae (ML) or goat anti-rabbit IgG, and a lateral flow (LF) assay based on these probes (UCNPs-ES/goat anti-rabbit IgG) was developed for the rapid and sensitive detection of anti-T. spiralis IgG antibodies in pig serum. The assay is named the UPT-LF-ES assay. In addition, the probes were characterized, and the assay was optimized. A cut-off threshold of the assay was also identified by using 169 known negative pig samples. Performance of the assay to T. spiralis with different infective numbers, cross-reactivity with other parasitic infections, the single-blinded experiment, and coincidence were evaluated with the assay.
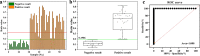
Results: The UPT-LF-ES assay was successfully constructed and optimized based on the probes of UCNPs-ES/goat anti-rabbit IgG. In the pigs infected with 100, 1000, and 10,000 ML, positive results were first presented at 35 days post-infection (dpi), 30 dpi, and 25 dpi, respectively. The assay had no cross-reaction with other parasitic infections. A single-blinded experiment indicated that the sensitivity and specificity of the UPT-LF-ES assay were 100% and 100%, respectively, the area under the receiver operating characteristic (ROC) curve was 1.000. In addition, the value detected by the UPT-LF-ES assay was significantly different between positive and negative samples. Moreover, compared with the "gold standard" magnetic stirrer method, the coincidence rate of the UPT-LF-ES assay was 87.27%, and the kappa (K) coefficient was 0.7454, showing a substantial agreement.
Conclusions: The UPT-LF-ES assay is a useful point-of-care test (POCT) with T. spiralis in the detection of pig, which contributes to preventing human trichinellosis.
Keywords: Excretory-secretory antigens; Lateral flow assay; Serodiagnosis; Trichinella spiralis; Upconverting phosphor technology.
© 2021. The Author(s).
Conflict of interest statement
All authors declare that they have no competing interests.
Figures






Similar articles
-
[The usefulness of ELISA test for early serological detection of Trichinella spp. infection in pigs].Wiad Parazytol. 2007;53(2):149-51. Wiad Parazytol. 2007. PMID: 17912813 Polish.
-
[The influence of the procedure of excretory-secretory L1 trichinella spiralis antigen preparation on the efficiency of an ELISA test in pigs].Wiad Parazytol. 2006;52(3):219-30. Wiad Parazytol. 2006. PMID: 17432246 Polish.
-
Development of a rapid and sensitive immunochromatographic strip based on EuNPs-ES fluorescent probe for the detection of early Trichinella spiralis-specific IgG antibody in pigs.Vet Res. 2021 Jun 11;52(1):85. doi: 10.1186/s13567-021-00951-9. Vet Res. 2021. PMID: 34116710 Free PMC article.
-
Vaccines against Trichinella spiralis: Progress, challenges and future prospects.Transbound Emerg Dis. 2018 Dec;65(6):1447-1458. doi: 10.1111/tbed.12917. Epub 2018 Jun 6. Transbound Emerg Dis. 2018. PMID: 29873198 Review.
-
Induction of protection in murine experimental models against Trichinella spiralis: an up-to-date review.J Helminthol. 2015 Sep;89(5):526-39. doi: 10.1017/S0022149X15000140. Epub 2015 Mar 12. J Helminthol. 2015. PMID: 25761655 Review.
Cited by
-
Simultaneous detection of circulating tumor DNAs using a SERS-based lateral flow assay biosensor for point-of-care diagnostics of head and neck cancer.Biomed Opt Express. 2022 Jul 5;13(8):4102-4117. doi: 10.1364/BOE.463612. eCollection 2022 Aug 1. Biomed Opt Express. 2022. PMID: 36032568 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

