Safety, Virologic Efficacy, and Pharmacokinetics of CT-P59, a Neutralizing Monoclonal Antibody Against SARS-CoV-2 Spike Receptor-Binding Protein: Two Randomized, Placebo-Controlled, Phase I Studies in Healthy Individuals and Patients With Mild SARS-CoV-2 Infection
- PMID: 34551869
- PMCID: PMC8380488
- DOI: 10.1016/j.clinthera.2021.08.009
Safety, Virologic Efficacy, and Pharmacokinetics of CT-P59, a Neutralizing Monoclonal Antibody Against SARS-CoV-2 Spike Receptor-Binding Protein: Two Randomized, Placebo-Controlled, Phase I Studies in Healthy Individuals and Patients With Mild SARS-CoV-2 Infection
Abstract
Purpose: Neutralizing antibodies can reduce SARS-CoV-2 cellular entry, viral titers, and pathologic damage. CT-P59 (regdanvimab), a SARS-CoV-2 neutralizing monoclonal antibody, was examined in 2 randomized, double-blind, placebo-controlled, single ascending dose, Phase I studies.
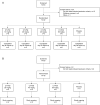
Methods: In study 1.1, healthy adults were sequentially enrolled to receive CT-P59 10, 20, 40, or 80 mg/kg or placebo. In study 1.2, adult patients with mild SARS-CoV-2 infection were enrolled to receive CT-P59 20, 40, or 80 mg/kg or placebo. Primary objectives of both studies were safety and tolerability up to day 14 after infusion. Secondary end points included pharmacokinetic properties. Study 1.2 also measured virology and clinical efficacy.
Findings: Thirty-two individuals were randomized to study 1.1 (6 per CT-P59 dose cohort and 8 in the placebo cohort). By day 14 after infusion, adverse events (AEs) were reported in 2 individuals receiving CT-P59 20 mg/kg (headache and elevated C-reactive protein levels) and 1 receiving CT-P59 40 mg/kg (pyrexia) (all Common Terminology Criteria for Adverse Events grade 1). In study 1.2, 18 patients were randomized (5 per dose cohort and 3 in the placebo cohort). Sixteen AEs were reported in 10 patients receiving CT-P59. No AEs in either study led to study discontinuation. Greater reductions in viral titers were reported with CT-P59 than placebo in those with maximum titers >105 copies/mL. Mean time to recovery was 3.39 versus 5.25 days.
Implications: CT-P59 exhibited a promising safety profile in healthy individuals and patients with mild SARS-CoV-2 infection, with potential antiviral and clinical efficacy in patients with mild SARS-CoV-2 infection. ClinicalTrials.gov identifier: NCT04525079 (study 1.1) and NCT04593641 (study 1.2).
Keywords: COVID-19; CT-P59; SARS-CoV-2; neutralizing monoclonal antibody; regdanvimab.
Copyright © 2021. Published by Elsevier Inc.
Figures



References
-
- Johns Hopkins Coronavirus Research Center . JHU; 2021. COVID-19 dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University.https://coronavirus.jhu.edu/map.html accessed 1 Jul 2021.
-
- World Health Organization . 2021. WHO Coronavirus Disease (COVID-19) Dashboard.https://covid19.who.int/ accessed 1 Jul 2021. - PubMed
-
- Wiersinga WJ, Rhodes A, Cheng AC, et al. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324:782–793. - PubMed
-
- Liu L, Wang P, Nair MS, et al. Potent neutralizing antibodies against multiple epitopes on SARS-CoV-2 spike. Nature. 2020;584:450–456. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

