Use of metformin to prolong gestation in preterm pre-eclampsia: randomised, double blind, placebo controlled trial
- PMID: 34551918
- PMCID: PMC8457042
- DOI: 10.1136/bmj.n2103
Use of metformin to prolong gestation in preterm pre-eclampsia: randomised, double blind, placebo controlled trial
Abstract
Objective: To evaluate whether extended release metformin could be used to prolong gestation in women being expectantly managed for preterm pre-eclampsia.
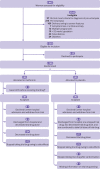
Design: Randomised, double blind, placebo controlled trial.
Setting: Referral hospital in Cape Town, South Africa.
Participants: 180 women with preterm pre-eclampsia between 26+0 to 31+6 weeks' gestation undergoing expectant management: 90 were randomised to extended release metformin and 90 to placebo.
Intervention: 3 g of oral extended release metformin or placebo daily, in divided doses, until delivery.
Main outcome measure: The primary outcome was prolongation of gestation.
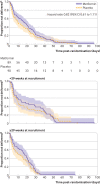
Results: Of 180 participants, one woman delivered before taking any trial drug. The median time from randomisation to delivery was 17.7 days (interquartile range 5.4-29.4 days; n=89) in the metformin arm and 10.1 (3.7-24.1; n=90) days in the placebo arm, a median difference of 7.6 days (geometric mean ratio 1.39, 95% confidence interval 0.99 to 1.95; P=0.057). Among those who continued to take the trial drug at any dose, the median prolongation of gestation in the metformin arm was 17.5 (interquartile range 5.4-28.7; n=76) days compared with 7.9 (3.0-22.2; n=74) days in the placebo arm, a median difference of 9.6 days (geometric mean ratio 1.67, 95% confidence interval 1.16 to 2.42). Among those who took the full dosage, the median prolongation of gestation in the metformin arm was 16.3 (interquartile range 4.8-28.8; n=40) days compared with 4.8 (2.5-15.4; n=61) days in the placebo arm, a median difference of 11.5 days (geometric mean ratio 1.85, 95% confidence interval 1.14 to 2.88). Composite maternal, fetal, and neonatal outcomes and circulating concentrations of soluble fms-like tyrosine kinase-1, placental growth factor, and soluble endoglin did not differ. In the metformin arm, birth weight increased non-significantly and length of stay decreased in the neonatal nursery. No serious adverse events related to trial drugs were observed, although diarrhoea was more common in the metformin arm.
Conclusions: This trial suggests that extended release metformin can prolong gestation in women with preterm pre-eclampsia, although further trials are needed. It provides proof of concept that treatment of preterm pre-eclampsia is possible.
Trial registration: Pan African Clinical Trial Registry PACTR201608001752102 https://pactr.samrc.ac.za/.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: support from the Mercy Health Foundation, Peter Joseph Pappas research grant programme, Preeclampsia Foundation, and South African Medical Research Council self-initiated research grants programme; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Figures
References
-
- Moodley J. Maternal deaths due to hypertensive disorders of pregnancy: data from the 2014-2016 Saving Mothers’ Report. Obstet Gynaecol Forum 2018;28:28-32.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
