Neurobehavioural comorbidities of epilepsy: towards a network-based precision taxonomy
- PMID: 34552218
- PMCID: PMC8900353
- DOI: 10.1038/s41582-021-00555-z
Neurobehavioural comorbidities of epilepsy: towards a network-based precision taxonomy
Abstract
Cognitive and behavioural comorbidities are prevalent in childhood and adult epilepsies and impose a substantial human and economic burden. Over the past century, the classic approach to understanding the aetiology and course of these comorbidities has been through the prism of the medical taxonomy of epilepsy, including its causes, course, characteristics and syndromes. Although this 'lesion model' has long served as the organizing paradigm for the field, substantial challenges to this model have accumulated from diverse sources, including neuroimaging, neuropathology, neuropsychology and network science. Advances in patient stratification and phenotyping point towards a new taxonomy for the cognitive and behavioural comorbidities of epilepsy, which reflects the heterogeneity of their clinical presentation and raises the possibility of a precision medicine approach. As we discuss in this Review, these advances are informing the development of a revised aetiological paradigm that incorporates sophisticated neurobiological measures, genomics, comorbid disease, diversity and adversity, and resilience factors. We describe modifiable risk factors that could guide early identification, treatment and, ultimately, prevention of cognitive and broader neurobehavioural comorbidities in epilepsy and propose a road map to guide future research.
© 2021. Springer Nature Limited.
Figures

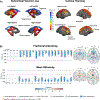
 Subcortical volume (left) and cortical thickness (right) abnormalities shared across all epilepsy syndromes in the ENIGMA-Epilepsy meta-analysis. Coloured bar represents Cohen’s d effect size estimates for case–control differences in each subcortical or cortical region. Red and yellow shading depicts regions with greater volume loss or thinning in patients relative to controls, whereas blue shading represents regions with higher volume relative to controls. Patients with epilepsy had lower volumes of the bilateral thalami and hippocampi and right pallidum relative to controls, and increased volume of the lateral ventricles. The patients also showed cortical thinning in the precentral and paracentral gyri bilaterally and in the left prefrontal, superior parietal and cuneus. b
Subcortical volume (left) and cortical thickness (right) abnormalities shared across all epilepsy syndromes in the ENIGMA-Epilepsy meta-analysis. Coloured bar represents Cohen’s d effect size estimates for case–control differences in each subcortical or cortical region. Red and yellow shading depicts regions with greater volume loss or thinning in patients relative to controls, whereas blue shading represents regions with higher volume relative to controls. Patients with epilepsy had lower volumes of the bilateral thalami and hippocampi and right pallidum relative to controls, and increased volume of the lateral ventricles. The patients also showed cortical thinning in the precentral and paracentral gyri bilaterally and in the left prefrontal, superior parietal and cuneus. b
 White matter microstructural differences across 38 fibre tracts for the ‘all epilepsies’ cohort compared with controls. All values represent Cohen’s d effect size estimates for differences in fractional anisotropy and mean diffusivity between each patient group and healthy controls. Positive effect sizes reflect diffusion values greater than controls and negative effect sizes represent values lower than controls. The y and z values represent the slice number for the coronal and axial planes, respectively. Across all epilepsies, the greatest effects on fractional anisotropy were observed in the body and genu of the corpus callosum, external capsule, cingulum and corona radiata. The greatest effects on mean diffusivity were observed in the external capsule, anterior corona radiata and superior longitudinal fasciculus. Part a reprinted with permission from ref.. Part b adapted with permission from ref..
White matter microstructural differences across 38 fibre tracts for the ‘all epilepsies’ cohort compared with controls. All values represent Cohen’s d effect size estimates for differences in fractional anisotropy and mean diffusivity between each patient group and healthy controls. Positive effect sizes reflect diffusion values greater than controls and negative effect sizes represent values lower than controls. The y and z values represent the slice number for the coronal and axial planes, respectively. Across all epilepsies, the greatest effects on fractional anisotropy were observed in the body and genu of the corpus callosum, external capsule, cingulum and corona radiata. The greatest effects on mean diffusivity were observed in the external capsule, anterior corona radiata and superior longitudinal fasciculus. Part a reprinted with permission from ref.. Part b adapted with permission from ref..


References
-
- World Health Organization. Epilepsy: A public health imperative. World Health Organization, https://www.who.int/publications-detail-redirect/epilepsy-a-public-healt... (2019).
-
- Venne J. et al. International consortium for personalized medicine: an international survey about the future of personalized medicine. Per Med 17, 89–100 (2020). - PubMed
-
- Jameson JL & Longo DL Precision medicine--personalized, problematic, and promising. N Engl J Med 372, 2229–2234 (2015). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

