Dopamine facilitates associative memory encoding in the entorhinal cortex
- PMID: 34552245
- PMCID: PMC8744500
- DOI: 10.1038/s41586-021-03948-8
Dopamine facilitates associative memory encoding in the entorhinal cortex
Abstract
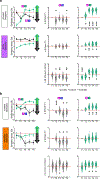
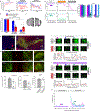
Mounting evidence shows that dopamine in the striatum is critically involved in reward-based reinforcement learning1,2. However, it remains unclear how dopamine reward signals influence the entorhinal-hippocampal circuit, another brain network that is crucial for learning and memory3-5. Here, using cell-type-specific electrophysiological recording6, we show that dopamine signals from the ventral tegmental area and substantia nigra control the encoding of cue-reward association rules in layer 2a fan cells of the lateral entorhinal cortex (LEC). When mice learned novel olfactory cue-reward associations using a pre-learned association rule, spike representations of LEC fan cells grouped newly learned rewarded cues with a pre-learned rewarded cue, but separated them from a pre-learned unrewarded cue. Optogenetic inhibition of fan cells impaired the learning of new associations while sparing the retrieval of pre-learned memory. Using fibre photometry, we found that dopamine sends novelty-induced reward expectation signals to the LEC. Inhibition of LEC dopamine signals disrupted the associative encoding of fan cells and impaired learning performance. These results suggest that LEC fan cells represent a cognitive map of abstract task rules, and that LEC dopamine facilitates the incorporation of new memories into this map.
© 2021. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Figures












References
-
- Squire LR Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychological review 99, 195–231 (1992). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

