Umami-induced obesity and metabolic syndrome is mediated by nucleotide degradation and uric acid generation
- PMID: 34552272
- PMCID: PMC9987717
- DOI: 10.1038/s42255-021-00454-z
Umami-induced obesity and metabolic syndrome is mediated by nucleotide degradation and uric acid generation
Abstract
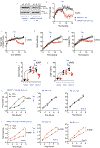
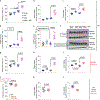
Umami refers to the savoury taste that is mediated by monosodium glutamate (MSG) and enhanced by inosine monophosphate and other nucleotides. Umami foods have been suggested to increase the risk for obesity and metabolic syndrome but the mechanism is not understood. Here we show that MSG induces obesity, hypothalamic inflammation and central leptin resistance in male mice through the induction of AMP deaminase 2 and purine degradation. Mice lacking AMP deaminase 2 in both hepatocytes and neurons are protected from MSG-induced metabolic syndrome. This protection can be overcome by supplementation with inosine monophosphate, most probably owing to its degradation to uric acid as the effect can be blocked with allopurinol. Thus, umami foods induce obesity and metabolic syndrome by engaging the same purine nucleotide degradation pathway that is also activated by fructose and salt consumption. We suggest that the three tastes-sweet, salt and umami-developed to encourage food intake to facilitate energy storage and survival but drive obesity and diabetes in the setting of excess intake through similar mechanisms.
© 2021. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Competing interests
M.A.L. and R.J.J. are inventors in two patents (nos. 13/814,568 and 62/473,005) related to the blockade of fructokinase to treat metabolic syndrome. M.A.L. and R.J.J. are founders and members of Colorado Research Partners, a company dedicated to the generation of fructokinase inhibitors. The other authors declare no competing interests.
Figures




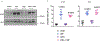







Comment in
-
Unravelling umami-induced obesity.Nat Rev Endocrinol. 2021 Dec;17(12):708. doi: 10.1038/s41574-021-00586-y. Nat Rev Endocrinol. 2021. PMID: 34663929 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

