Albuca Bracteate Polysaccharides Synergistically Enhance the Anti-Tumor Efficacy of 5-Fluorouracil Against Colorectal Cancer by Modulating β-Catenin Signaling and Intestinal Flora
- PMID: 34552494
- PMCID: PMC8450769
- DOI: 10.3389/fphar.2021.736627
Albuca Bracteate Polysaccharides Synergistically Enhance the Anti-Tumor Efficacy of 5-Fluorouracil Against Colorectal Cancer by Modulating β-Catenin Signaling and Intestinal Flora
Abstract
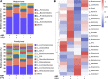
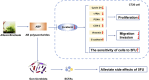
The first-line treatment for colorectal cancer (CRC) is 5-fluorouracil (5-FU). However, the efficacy of this treatment is sometimes limited owing to chemoresistance as well as treatment-associated intestinal mucositis and other adverse events. Growing evidence suggests that certain phytochemicals have therapeutic and cancer-preventing properties. Further, the synergistic interactions between many such plant-derived products and chemotherapeutic drugs have been linked to improved therapeutic efficacy. Polysaccharides extracted from Albuca bracteata (Thunb.) J.C.Manning and Goldblatt (ABP) have been reported to exhibit anti-oxidant, anti-inflammatory, and anti-tumor properties. In this study, murine CRC cells (CT26) and a murine model of CRC were used to examine the anti-tumor properties of ABP and explore the mechanism underlying the synergistic interactions between ABP and 5-FU. Our results revealed that ABP could inhibit tumor cell proliferation, invasion, and migratory activity in vitro and inhibited tumor progression in vivo by suppressing β-catenin signaling. Additionally, treatment with a combination of ABP and 5-FU resulted in better outcomes than treatment with either agent alone. Moreover, this combination therapy resulted in the specific enrichment of Ruminococcus, Anaerostipes, and Oscillospira in the intestinal microbiota and increased fecal short-chain fatty acid (SCFA) levels (acetic acid, propionic acid, and butyric acid). The improvement in the intestinal microbiota and the increase in beneficial SCFAs contributed to enhanced therapeutic outcomes and reduced the adverse effects of 5-FU. Together, these data suggest that ABP exhibits anti-neoplastic activity and can effectively enhance the efficacy of 5-FU in CRC treatment. Therefore, further research on the application of ABP in the development of novel anti-tumor drugs and adjuvant compounds is warranted and could improve the outcomes of CRC patients.
Keywords: 5-fluorouracil; colorectal cancer; gut microbiota; polysaccharide; short-chain fatty acids; β-catenin.
Copyright © 2021 Yuan, Xue, Tan, Yang, Qin, Bao, Li, Pan, Jiang, Wang, Lou, Jiang and Du.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures








References
LinkOut - more resources
Full Text Sources
