GLP-1 and Underlying Beneficial Actions in Alzheimer's Disease, Hypertension, and NASH
- PMID: 34552561
- PMCID: PMC8450670
- DOI: 10.3389/fendo.2021.721198
GLP-1 and Underlying Beneficial Actions in Alzheimer's Disease, Hypertension, and NASH
Abstract
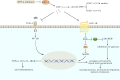
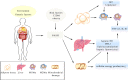
GLP-1 is derived from intestinal L cells, which takes effect through binding to GLP-1R and is inactivated by the enzyme dipeptidyl peptidase-4 (DPP-4). Since its discovery, GLP-1 has emerged as an incretin hormone for its facilitation in insulin release and reduction of insulin resistance (IR). However, GLP-1 possesses broader pharmacological effects including anti-inflammation, neuro-protection, regulating blood pressure (BP), and reducing lipotoxicity. These effects are interconnected to the physiological and pathological processes of Alzheimer's disease (AD), hypertension, and non-alcoholic steatohepatitis (NASH). Currently, the underlying mechanism of these effects is still not fully illustrated and a better understanding of them may help identify promising therapeutic targets of AD, hypertension, and NASH. Therefore, we focus on the biological characteristics of GLP-1, render an overview of the mechanism of GLP-1 effects in diseases, and investigate the potential of GLP-1 analogues for the treatment of related diseases in this review.
Keywords: Alzheimer’s disease; DPP-4; GLP-1; blood pressure; non-alcoholic steatohepatitis; signaling pathway.
Copyright © 2021 Li, Gao, Guo, Wang, Hua, Gao, Shang, Lu and Xu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

