Structural Analysis of SARS-CoV-2 ORF8 Protein: Pathogenic and Therapeutic Implications
- PMID: 34552615
- PMCID: PMC8450498
- DOI: 10.3389/fgene.2021.693227
Structural Analysis of SARS-CoV-2 ORF8 Protein: Pathogenic and Therapeutic Implications
Abstract
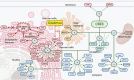
Current therapeutic strategies and vaccines against SARS-CoV-2 are mainly focused on the Spike protein despite there are other viral proteins with important roles in COVID-19 pathogenicity. For example, ORF8 restructures vesicular trafficking in the host cell, impacts intracellular immunity through the IFN-I signaling, and growth pathways through the mitogen-activated protein kinases (MAPKs). In this mini-review, we analyze the main structural similarities of ORF8 with immunological molecules such as IL-1, contributing to the immunological deregulation observed in COVID-19. We also propose that the blockage of some effector functions of ORF8 with Rapamycin, such as the mTORC1 activation through MAPKs 40 pathway, with Rapamycin, can be a promising approach to reduce COVID-19 mortality.
Keywords: COVID-19; COVID-19 therapeutics; ORF8; SARS-CoV-2; structural biology.
Copyright © 2021 Valcarcel, Bensussen, Álvarez-Buylla and Díaz.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Accurate Diagnosis of COVID-19 by a Novel Immunogenic Secreted SARS-CoV-2 orf8 Protein.mBio. 2020 Oct 20;11(5):e02431-20. doi: 10.1128/mBio.02431-20. mBio. 2020. PMID: 33082264 Free PMC article.
-
SARS-CoV-2 ORF8: One protein, seemingly one structure, and many functions.Front Immunol. 2022 Oct 24;13:1035559. doi: 10.3389/fimmu.2022.1035559. eCollection 2022. Front Immunol. 2022. PMID: 36353628 Free PMC article. Review.
-
SARS-CoV-2 ORF8 reshapes the ER through forming mixed disulfides with ER oxidoreductases.Redox Biol. 2022 Aug;54:102388. doi: 10.1016/j.redox.2022.102388. Epub 2022 Jun 28. Redox Biol. 2022. PMID: 35792438 Free PMC article.
-
SARS-CoV-2 ORF8 dimerization and binding mode analysis with class I MHC: computational approaches to identify COVID-19 inhibitors.Brief Funct Genomics. 2023 Apr 13;22(2):227-240. doi: 10.1093/bfgp/elac046. Brief Funct Genomics. 2023. PMID: 36827221
-
SARS-CoV-2 ORF8 and SARS-CoV ORF8ab: Genomic Divergence and Functional Convergence.Pathogens. 2020 Aug 20;9(9):677. doi: 10.3390/pathogens9090677. Pathogens. 2020. PMID: 32825438 Free PMC article. Review.
Cited by
-
SARS-CoV-2 Accessory Protein ORF8 Targets the Dimeric IgA Receptor pIgR.Viruses. 2024 Jun 22;16(7):1008. doi: 10.3390/v16071008. Viruses. 2024. PMID: 39066171 Free PMC article.
-
Alignment of human KAT2A (GCN5) histone acetyltransferase and SARS-CoV-2 Orf8 viral proteins.Chronic Dis Transl Med. 2023 Sep;9(3):263-265. doi: 10.1002/cdt3.56. Epub 2022 Dec 30. Chronic Dis Transl Med. 2023. PMID: 36721422 Free PMC article. No abstract available.
-
ORF8 and Health Complications of COVID-19 in Down Syndrome Patients.Front Genet. 2022 Feb 9;13:830426. doi: 10.3389/fgene.2022.830426. eCollection 2022. Front Genet. 2022. PMID: 35222544 Free PMC article. No abstract available.
-
Applying the digital data and the bioinformatics tools in SARS-CoV-2 research.Comput Struct Biotechnol J. 2023 Oct 1;21:4697-4705. doi: 10.1016/j.csbj.2023.09.044. eCollection 2023. Comput Struct Biotechnol J. 2023. PMID: 37841328 Free PMC article. Review.
-
Essential contribution of the JAK/STAT pathway to carcinogenesis, lytic infection of herpesviruses and pathogenesis of COVID‑19 (Review).Mol Med Rep. 2024 Mar;29(3):39. doi: 10.3892/mmr.2024.13163. Epub 2024 Jan 19. Mol Med Rep. 2024. PMID: 38240082 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

