Complexity of skeletal muscle degeneration: multi-systems pathophysiology and organ crosstalk in dystrophinopathy
- PMID: 34553265
- PMCID: PMC8599371
- DOI: 10.1007/s00424-021-02623-1
Complexity of skeletal muscle degeneration: multi-systems pathophysiology and organ crosstalk in dystrophinopathy
Abstract
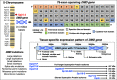
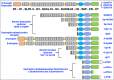
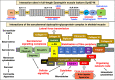
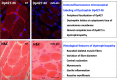
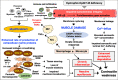
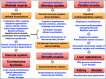
Duchenne muscular dystrophy is a highly progressive muscle wasting disorder due to primary abnormalities in one of the largest genes in the human genome, the DMD gene, which encodes various tissue-specific isoforms of the protein dystrophin. Although dystrophinopathies are classified as primary neuromuscular disorders, the body-wide abnormalities that are associated with this disorder and the occurrence of organ crosstalk suggest that a multi-systems pathophysiological view should be taken for a better overall understanding of the complex aetiology of X-linked muscular dystrophy. This article reviews the molecular and cellular effects of deficiency in dystrophin isoforms in relation to voluntary striated muscles, the cardio-respiratory system, the kidney, the liver, the gastrointestinal tract, the nervous system and the immune system. Based on the establishment of comprehensive biomarker signatures of X-linked muscular dystrophy using large-scale screening of both patient specimens and genetic animal models, this article also discusses the potential usefulness of novel disease markers for more inclusive approaches to differential diagnosis, prognosis and therapy monitoring that also take into account multi-systems aspects of dystrophinopathy. Current therapeutic approaches to combat muscular dystrophy are summarised.
Keywords: Duchenne muscular dystrophy; Dystrophin; Fibrosis; Inflammation; Muscle degeneration; Organ crosstalk.
© 2021. The Author(s).
Figures

Similar articles
-
Emerging proteomic biomarkers of X-linked muscular dystrophy.Expert Rev Mol Diagn. 2019 Aug;19(8):739-755. doi: 10.1080/14737159.2019.1648214. Epub 2019 Aug 2. Expert Rev Mol Diagn. 2019. PMID: 31359811 Review.
-
Histopathology of Duchenne muscular dystrophy in correlation with changes in proteomic biomarkers.Histol Histopathol. 2022 Feb;37(2):101-116. doi: 10.14670/HH-18-403. Epub 2021 Dec 7. Histol Histopathol. 2022. PMID: 34873679 Review.
-
Proteomic profiling of fatty acid binding proteins in muscular dystrophy.Expert Rev Proteomics. 2020 Feb;17(2):137-148. doi: 10.1080/14789450.2020.1732214. Epub 2020 Feb 24. Expert Rev Proteomics. 2020. PMID: 32067530 Review.
-
Proteomic analysis of the sarcolemma-enriched fraction from dystrophic mdx-4cv skeletal muscle.J Proteomics. 2019 Jan 16;191:212-227. doi: 10.1016/j.jprot.2018.01.015. Epub 2018 Feb 2. J Proteomics. 2019. PMID: 29408692
-
A novel canine model for Duchenne muscular dystrophy (DMD): single nucleotide deletion in DMD gene exon 20.Skelet Muscle. 2018 May 29;8(1):16. doi: 10.1186/s13395-018-0162-1. Skelet Muscle. 2018. PMID: 29843823 Free PMC article.
Cited by
-
CB2 Receptor as Emerging Anti-Inflammatory Target in Duchenne Muscular Dystrophy.Int J Mol Sci. 2023 Feb 7;24(4):3345. doi: 10.3390/ijms24043345. Int J Mol Sci. 2023. PMID: 36834757 Free PMC article.
-
miR-103-3p Regulates the Proliferation and Differentiation of C2C12 Myoblasts by Targeting BTG2.Int J Mol Sci. 2023 Oct 18;24(20):15318. doi: 10.3390/ijms242015318. Int J Mol Sci. 2023. PMID: 37894995 Free PMC article.
-
Histological and Histochemical Microscopy Used to Verify 2D-DIGE Pathoproteomics.Methods Mol Biol. 2023;2596:465-480. doi: 10.1007/978-1-0716-2831-7_31. Methods Mol Biol. 2023. PMID: 36378457
-
Verification of Protein Changes Determined by 2D-DIGE Based Proteomics Using Immunofluorescence Microscopy.Methods Mol Biol. 2023;2596:445-464. doi: 10.1007/978-1-0716-2831-7_30. Methods Mol Biol. 2023. PMID: 36378456
-
Proteomic profiling of impaired excitation-contraction coupling and abnormal calcium handling in muscular dystrophy.Proteomics. 2022 Dec;22(23-24):e2200003. doi: 10.1002/pmic.202200003. Epub 2022 Aug 8. Proteomics. 2022. PMID: 35902360 Free PMC article. Review.
References
-
- Alderton JM, Steinhardt RA. Calcium influx through calcium leak channels is responsible for the elevated levels of calcium-dependent proteolysis in dystrophic myotubes. J Biol Chem. 2000;275:9452–9460. - PubMed
-
- Al-Khalili Szigyarto C. Duchenne Muscular Dystrophy: recent advances in protein biomarkers and the clinical application. Expert Rev Proteomics. 2020;17:365–375. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

