Predictors of Ocrelizumab Effectiveness in Patients with Multiple Sclerosis
- PMID: 34553320
- PMCID: PMC8457546
- DOI: 10.1007/s13311-021-01104-8
Predictors of Ocrelizumab Effectiveness in Patients with Multiple Sclerosis
Abstract
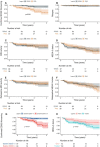
Data regarding effectiveness and safety of ocrelizumab in the post-marking setting are lacking. The aim of our study was to provide effectiveness and safety data of ocrelizumab treatment in patients with relapsing-remitting (RR-) and progressive multiple sclerosis (PMS) and to evaluate clinical and immunological predictors of early treatment response. In this single-center prospective observational study, we investigated effectiveness outcomes (time-to-confirmed disability worsening, time-to-first relapse, time-to-first evidence of MRI activity and time-to-first evidence of disease activity), clinical and immunological predictors of early treatment response, and incidence of adverse events (AEs). One hundred and fifty-three subjects were included (93 RRMS; 84 females). Median follow-up was 1.9 (1.3-2.7). At 2-year follow-up (FU), disability worsening-free survival were 90.5%, 64.7%, and 68.8% for RRMS, primary-progressive MS (PPMS), and secondary-progressive MS (SPMS) patients, respectively. At 2-year FU, 67.1%, 72.7%, and 81.3% of patients with RRMS, PPMS, and SPMS were free of MRI activity, with NEDA-3 percentages of 62.1%, 54.6%, and 55.1%, respectively. Lower baseline EDSS was independently associated with a reduced risk of disability worsening (HR(95%CI) = 1.45(1.05-2.00), p = 0.024) and previous treatment exposure was independently associated with increased probability of radiological activity (HR = 2.53(1.05-6.10), p = 0.039). At 6-month FU, CD8 + cell decrease was less pronounced in patients with inflammatory activity (p = 0.022). Six patients (3.9%) discontinued ocrelizumab due to severe AEs. Our findings suggest that ocrelizumab is an effective treatment in real-world patients with RRMS and PMS, with a manageable safety profile. Better outcomes were observed in treatment-naïve patients and in patients with a low baseline disability level. Depletion of CD8 + cells could underlie early therapeutic effects of ocrelizumab.
Keywords: Advanced multiple sclerosis; CD8; Highly active multiple sclerosis; Multiple sclerosis; Ocrelizumab.
© 2021. The Author(s).
Figures
References
-
- Hauser SL, Bar-Or A, Comi G, Giovannoni G, Hartung H-P, Hemmer B, et al. Ocrelizumab versus Interferon Beta-1a in Relapsing Multiple Sclerosis. N Engl J Med [Internet]. 2017 Jan 19;376(3):221–34. Available from: 10.1056/NEJMoa1601277 - PubMed
-
- Montalban X, Hauser SL, Kappos L, Arnold DL, Bar-Or A, Comi G, et al. Ocrelizumab versus Placebo in Primary Progressive Multiple Sclerosis. N Engl J Med [Internet]. 2017 Jan 19;376(3):209–20. Available from: 10.1056/NEJMoa1606468 - PubMed
-
- Wolinsky JS, Arnold DL, Brochet B, Hartung H-P, Montalban X, Naismith RT, et al. Long-term follow-up from the ORATORIO trial of ocrelizumab for primary progressive multiple sclerosis: a post-hoc analysis from the ongoing open-label extension of the randomised, placebo-controlled, phase 3 trial. Lancet Neurol [Internet]. 2020;1–12. Available from: 10.1016/S1474-4422(20)30342-2 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

