Kinetic Analysis of a Cysteine-Derived Thiyl-Catalyzed Asymmetric Vinylcyclopropane Cycloaddition Reflects Numerous Attractive Noncovalent Interactions
- PMID: 34553915
- PMCID: PMC8962000
- DOI: 10.1021/jacs.1c07323
Kinetic Analysis of a Cysteine-Derived Thiyl-Catalyzed Asymmetric Vinylcyclopropane Cycloaddition Reflects Numerous Attractive Noncovalent Interactions
Abstract
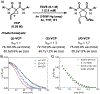
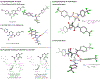
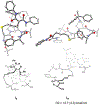
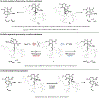
Kinetic studies of a vinylcyclopropane (VCP) cycloaddition, catalyzed by peptide-based thiyl radicals, are described. Reactions were analyzed by using reaction progress kinetic analysis, revealing that ring-opening of the VCP is both rate- and enantio-determining. These conclusions are further corroborated by studies involving racemic and enantiopure VCP starting material. Noncovalent interactions play key roles throughout: both the peptide catalyst and VCP exhibit unproductive self-aggregation, which appears to be disrupted by binding between the catalyst and VCP. This in turn explains the requirement for the key catalyst feature, a substituent at the 4-position of the proline residue, which is required for both turnover/rate and selectivity.
Figures



References
-
- Knappe J; Elbert S; Frey M; Wagner AFV Pyruvate formase-lyase mechanism involving the protein-based glycyl radical. Biochem. Soc. Trans 1993, 21, 731–734. - PubMed
-
- Licht S; Gerfen GJ; Stubbe J Thiyl Radicals in Ribonucleotide Reductase. Science 1996, 271, 477–481. - PubMed
-
- Stubbe J; van der Donk WA Protein Radicals in Enzyme Catalysis. Chem. Rev 1998, 98, 705–762. - PubMed
-
- Dénès F; Pichowicz M; Povie G; Renaud P Thiyl Radicals in Organic Synthesis. Chem. Rev 2014, 114, 2587–2693, and references therein. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

