BTK operates a phospho-tyrosine switch to regulate NLRP3 inflammasome activity
- PMID: 34554188
- PMCID: PMC8480672
- DOI: 10.1084/jem.20201656
BTK operates a phospho-tyrosine switch to regulate NLRP3 inflammasome activity
Abstract
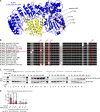
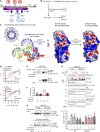
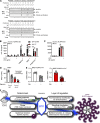
Activity of the NLRP3 inflammasome, a critical mediator of inflammation, is controlled by accessory proteins, posttranslational modifications, cellular localization, and oligomerization. How these factors relate is unclear. We show that a well-established drug target, Bruton's tyrosine kinase (BTK), affects several levels of NLRP3 regulation. BTK directly interacts with NLRP3 in immune cells and phosphorylates four conserved tyrosine residues upon inflammasome activation, in vitro and in vivo. Furthermore, BTK promotes NLRP3 relocalization, oligomerization, ASC polymerization, and full inflammasome assembly, probably by charge neutralization, upon modification of a polybasic linker known to direct NLRP3 Golgi association and inflammasome nucleation. As NLRP3 tyrosine modification by BTK also positively regulates IL-1β release, we propose BTK as a multifunctional positive regulator of NLRP3 regulation and BTK phosphorylation of NLRP3 as a novel and therapeutically tractable step in the control of inflammation.
© 2021 Bittner et al.
Conflict of interest statement
Disclosures: J.B. Kümmerle-Deschner reported grants from Novartis, personal fees from Novartis, grants from SOBI, and personal fees from SOBI outside the submitted work. B. Grimbacher reported grants from BMBF, grants from DFG, grants from several pharmaceutical companies, personal fees from several pharmaceutical companies, and grants from foundations outside the submitted work. E. Latz is co-founder and consultant to IFM Therapeutics. No other disclosures were reported.
Figures






References
-
- Carpenter, A.E., Jones T.R., Lamprecht M.R., Clarke C., Kang I.H., Friman O., Guertin D.A., Chang J.H., Lindquist R.A., Moffat J., et al. . 2006. CellProfiler: image analysis software for identifying and quantifying cell phenotypes. Genome Biol. 7:R100. 10.1186/gb-2006-7-10-r100 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

