Humoral and T-Cell Response to SARS-CoV-2 Vaccination in Patients With Multiple Sclerosis Treated With Ocrelizumab
- PMID: 34554197
- PMCID: PMC8461553
- DOI: 10.1001/jamaneurol.2021.3599
Humoral and T-Cell Response to SARS-CoV-2 Vaccination in Patients With Multiple Sclerosis Treated With Ocrelizumab
Abstract
Importance: B-cell-depleting therapies may affect the development of a protective immune response following vaccination. Understanding the ability to develop vaccine-specific immunity to COVID-19 in patients with multiple sclerosis (MS) treated with B-cell-depleting therapy is of importance for clinical decisions.
Objective: To assess SARS-CoV-2 vaccine-specific humoral and cellular responses in patients treated with ocrelizumab compared with healthy controls.
Design, setting, and participants: This single-center study performed at Hadassah Medical Center in Jerusalem, Israel, included patients with MS treated with ocrelizumab, healthy controls, and untreated patients with MS. Vaccination occurred between December 2020 and April 2021. Participants donated blood 2 to 4 and 2 to 8 weeks after the second vaccine dose for antibody and T-cell assessments, respectively.
Exposures: All participants received 2 doses of BNT162b2 vaccine (Pfizer/BioNTech) and completed the study.
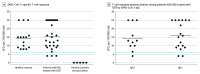
Main outcomes and measures: Proportion of patients treated with ocrelizumab with SARS-CoV-2-specific serology and/or T-cell responses following vaccination. All participants underwent SARS-CoV-2 antibody testing; 29 patients treated with ocrelizumab and 15 healthy controls had evaluation of SARS-CoV-2-specific T-cell responses.
Results: Of 112 participants, 49 (43.8%) had MS and were treated with ocrelizumab (33 [67.3%] female; mean [SD] age, 47.9 [13.3] years), 23 (20.5%) had MS and were not treated with disease-modifying therapies (18 [78.3%] female; mean [SD] age, 49 [13.4] years), and 40 (35.7%) were healthy controls (25 [62.5%] female; mean [SD] age, 45.3 [16] years). Twenty-six of 29 patients (89.7%) treated with ocrelizumab and 15 of 15 healthy controls (100%) had SARS-CoV-2-specific T cells following vaccination at similar levels (mean [SD], 15.4 [7.6] and 14.3 [6.3] spot-forming cells, respectively). Mean antibody titers and positive serology rate were lower in the group of patients treated with ocrelizumab (mean [SD] antibody titers and positive serology rate, 26.2 [49.2] and 376.5 [907.6] AU/mL; 10 of 40 [25%] and 20 of 49 [40.8%] for S1/S2 and receptor-binding domain, respectively) compared with healthy controls (mean [SD] antibody titers and positive serology rate, 283 [100] and 12 712 [9114] AU/mL; 100% S1/S2 and receptor-binding domain) and untreated patients (mean [SD] antibody titers and positive serology rate, 288.3 [113.8] and 10 877 [9476] AU/mL; 100% S1/S2 and receptor-binding domain), with positive association to time from ocrelizumab infusion (S1/S2: r = 0.7, P < .001; receptor-binding domain: r = 0.4, P = .04).
Conclusion and relevance: In this study, patients with MS who were treated with ocrelizumab generated comparable SARS-CoV-2-specific T-cell responses with healthy controls and had lower antibody response following vaccination. Given the potential role of T cells in protection from severe disease, this is reassuring and will help physicians develop consensus guidelines regarding MS treatment in the era of the COVID-19 pandemic.
Conflict of interest statement
Figures

Comment in
-
Response to SARS-CoV-2 vaccination in multiple sclerosis patients on disease modifying therapies.J Neurol. 2022 Apr;269(4):2259-2261. doi: 10.1007/s00415-022-11053-7. Epub 2022 Mar 14. J Neurol. 2022. PMID: 35286479 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

