Final Overall Survival Efficacy Results of Ivosidenib for Patients With Advanced Cholangiocarcinoma With IDH1 Mutation: The Phase 3 Randomized Clinical ClarIDHy Trial
- PMID: 34554208
- PMCID: PMC8461552
- DOI: 10.1001/jamaoncol.2021.3836
Final Overall Survival Efficacy Results of Ivosidenib for Patients With Advanced Cholangiocarcinoma With IDH1 Mutation: The Phase 3 Randomized Clinical ClarIDHy Trial
Abstract
Importance: Isocitrate dehydrogenase 1 (IDH1) variations occur in up to approximately 20% of patients with intrahepatic cholangiocarcinoma. In the ClarIDHy trial, progression-free survival as determined by central review was significantly improved with ivosidenib vs placebo.
Objective: To report the final overall survival (OS) results from the ClarIDHy trial, which aimed to demonstrate the efficacy of ivosidenib (AG-120)-a first-in-class, oral, small-molecule inhibitor of mutant IDH1-vs placebo for patients with unresectable or metastatic cholangiocarcinoma with IDH1 mutation.
Design, setting, and participants: This multicenter, randomized, double-blind, placebo-controlled, clinical phase 3 trial was conducted from February 20, 2017, to May 31, 2020, at 49 hospitals across 6 countries among patients aged 18 years or older with cholangiocarcinoma with IDH1 mutation whose disease progressed with prior therapy.
Interventions: Patients were randomized 2:1 to receive ivosidenib, 500 mg, once daily or matched placebo. Crossover from placebo to ivosidenib was permitted if patients had disease progression as determined by radiographic findings.
Main outcomes and measures: The primary end point was progression-free survival as determined by blinded independent radiology center (reported previously). Overall survival was a key secondary end point. The primary analysis of OS followed the intent-to-treat principle. Other secondary end points included objective response rate, safety and tolerability, and quality of life.
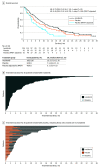
Results: Overall, 187 patients (median age, 62 years [range, 33-83 years]) were randomly assigned to receive ivosidenib (n = 126; 82 women [65%]; median age, 61 years [range, 33-80 years]) or placebo (n = 61; 37 women [61%]; median age, 63 years [range, 40-83 years]); 43 patients crossed over from placebo to ivosidenib. The primary end point of progression-free survival was reported elsewhere. Median OS was 10.3 months (95% CI, 7.8-12.4 months) with ivosidenib vs 7.5 months (95% CI, 4.8-11.1 months) with placebo (hazard ratio, 0.79 [95% CI, 0.56-1.12]; 1-sided P = .09). When adjusted for crossover, median OS with placebo was 5.1 months (95% CI, 3.8-7.6 months; hazard ratio, 0.49 [95% CI, 0.34-0.70]; 1-sided P < .001). The most common grade 3 or higher treatment-emergent adverse event (≥5%) reported in both groups was ascites (11 patients [9%] receiving ivosidenib and 4 patients [7%] receiving placebo). Serious treatment-emergent adverse events considered ivosidenib related were reported in 3 patients (2%). There were no treatment-related deaths. Patients receiving ivosidenib reported no apparent decline in quality of life compared with placebo.
Conclusions and relevance: This randomized clinical trial found that ivosidenib was well tolerated and resulted in a favorable OS benefit vs placebo, despite a high rate of crossover. These data, coupled with supportive quality of life data and a tolerable safety profile, demonstrate the clinical benefit of ivosidenib for patients with advanced cholangiocarcinoma with IDH1 mutation.
Trial registration: ClinicalTrials.gov Identifier: NCT02989857.
Conflict of interest statement
Figures
References
-
- Lamarca A, Palmer DH, Wasan HS, et al. . ABC-06: a randomised phase III, multi-centre, open-label study of active symptom control (ASC) alone or ASC with oxaliplatin/5-FU chemotherapy (ASC+mFOLFOX) for patients (pts) with locally advanced/metastatic biliary tract cancers (ABC) previously-treated with cisplatin/gemcitabine (CisGem) chemotherapy. J Clin Oncol. 2019;37(15)(suppl):abstract 4003. doi:10.1200/JCO.2019.37.15_suppl.4003 - DOI
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

