Prediction of Moxifloxacin Concentrations in Tuberculosis Patient Populations by Physiologically Based Pharmacokinetic Modeling
- PMID: 34554580
- PMCID: PMC9297990
- DOI: 10.1002/jcph.1972
Prediction of Moxifloxacin Concentrations in Tuberculosis Patient Populations by Physiologically Based Pharmacokinetic Modeling
Abstract
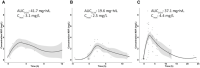
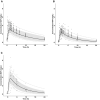
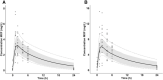
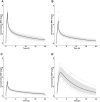
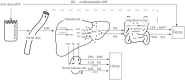
Moxifloxacin has an important role in the treatment of tuberculosis (TB). Unfortunately, coadministration with the cornerstone TB drug rifampicin results in suboptimal plasma exposure. We aimed to gain insight into the moxifloxacin pharmacokinetics and the interaction with rifampicin. Moreover, we provided a mechanistic framework to understand moxifloxacin pharmacokinetics. We developed a physiologically based pharmacokinetic model in Simcyp version 19, with available and newly generated in vitro and in vivo data, to estimate pharmacokinetic parameters of moxifloxacin alone and when administered with rifampicin. By combining these strategies, we illustrate that the role of P-glycoprotein in moxifloxacin transport is limited and implicate MRP2 as transporter of moxifloxacin-glucuronide followed by rapid hydrolysis in the gut. Simulations of multiple dose area under the plasma concentration-time curve (AUC) of moxifloxacin (400 mg once daily) with and without rifampicin (600 mg once daily) were in accordance with clinically observed data (predicted/observed [P/O] ratio of 0.87 and 0.80, respectively). Importantly, increasing the moxifloxacin dose to 600 mg restored the plasma exposure both in actual patients with TB as well as in our simulations. Furthermore, we extrapolated the single dose model to pediatric populations (P/O AUC ratios, 1.04-1.52) and the multiple dose model to children with TB (P/O AUC ratio, 1.51). In conclusion, our combined approach resulted in new insights into moxifloxacin pharmacokinetics and accurate simulations of moxifloxacin exposure with and without rifampicin. Finally, various knowledge gaps were identified, which may be considered as avenues for further physiologically based pharmacokinetic refinement.
Keywords: drug-drug interactions; modeling; moxifloxacin; physiologically based pharmacokinetics; tuberculosis.
© 2021 The Authors. The Journal of Clinical Pharmacology published by Wiley Periodicals LLC on behalf of American College of Clinical Pharmacology.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- WHO . Global tuberculosis report 2019. https://www.who.int/tb/publications/global_report/en/. Accessed December 11, 2019.
-
- Soman A, Honeybourne D, Andrews J, Jevons G, Wise R. Concentrations of moxifloxacin in serum and pulmonary compartments following a single 400 mg oral dose in patients undergoing fibre‐optic bronchoscopy. J Antimicrob Chemother. 1999;44(6):835‐838. - PubMed
-
- WHO consolidated guidelines on drug‐resistant tuberculosis treatment. Geneva: World Health Organization; 2019. Licence: CC BY‐NC‐SA 3.0 IGO. - PubMed
-
- Ruslami R, Ganiem AR, Dian S, et al. Intensified regimen containing rifampicin and moxifloxacin for tuberculous meningitis: an open‐label, randomised controlled phase 2 trial. Lancet Infect Dis. 2013;13(1):27‐35. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

