Late-in-life treadmill training rejuvenates autophagy, protein aggregate clearance, and function in mouse hearts
- PMID: 34554626
- PMCID: PMC8520717
- DOI: 10.1111/acel.13467
Late-in-life treadmill training rejuvenates autophagy, protein aggregate clearance, and function in mouse hearts
Abstract
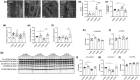
Protein quality control mechanisms decline during the process of cardiac aging. This enables the accumulation of protein aggregates and damaged organelles that contribute to age-associated cardiac dysfunction. Macroautophagy is the process by which post-mitotic cells such as cardiomyocytes clear defective proteins and organelles. We hypothesized that late-in-life exercise training improves autophagy, protein aggregate clearance, and function that is otherwise dysregulated in hearts from old vs. adult mice. As expected, 24-month-old male C57BL/6J mice (old) exhibited repressed autophagosome formation and protein aggregate accumulation in the heart, systolic and diastolic dysfunction, and reduced exercise capacity vs. 8-month-old (adult) mice (all p < 0.05). To investigate the influence of late-in-life exercise training, additional cohorts of 21-month-old mice did (old-ETR) or did not (old-SED) complete a 3-month progressive resistance treadmill running program. Body composition, exercise capacity, and soleus muscle citrate synthase activity improved in old-ETR vs. old-SED mice at 24 months (all p < 0.05). Importantly, protein expression of autophagy markers indicate trafficking of the autophagosome to the lysosome increased, protein aggregate clearance improved, and overall function was enhanced (all p < 0.05) in hearts from old-ETR vs. old-SED mice. These data provide the first evidence that a physiological intervention initiated late-in-life improves autophagic flux, protein aggregate clearance, and contractile performance in mouse hearts.
Keywords: aging; cardiac function; exercise; protein aggregates.
© 2021 The Authors. Aging Cell published by Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
None of the authors has any conflicts of interest to disclose.
Figures





References
-
- Benjamin, E. J. , Muntner, P. , Alonso, A. , Bittencourt, M. S. , Callaway, C. W. , Carson, A. P. , Chamberlain, A. M. , Chang, A. R. , Cheng, S. , Das, S. R. , Delling, F. N. , Djousse, L. , Elkind, M. S. V. , Ferguson, J. F. , Fornage, M. , Jordan, L. C. , Khan, S. S. , Kissela, B. M. , Knutson, K. L. , … Virani, S. S. (2019). Heart disease and stroke statistics‐2019 update: A report from the American Heart Association. Circulation, 139(10), e56–e528. 10.1161/CIR.0000000000000659 - DOI - PubMed
-
- Bharath, L. P. , Cho, J. M. , Park, S.‐K. , Ruan, T. , Li, Y. , Mueller, R. , Bean, T. , Reese, V. , Richardson, R. S. , Cai, J. , Sargsyan, A. , Pires, K. , Anandh Babu, P. V. , Boudina, S. , Graham, T. E. , Symons, J. D. (2017). Endothelial Cell Autophagy maintains shear stress‐induced nitric oxide generation via glycolysis‐dependent purinergic signaling to endothelial nitric oxide synthase. Arteriosclerosis, Thrombosis, and Vascular Biology, 37(9), 1646–1656. 10.1161/ATVBAHA.117.309510 - DOI - PMC - PubMed
-
- Bharath, L. P. , Ruan, T. , Li, Y. , Ravindran, A. , Wan, X. , Nhan, J. K. , Walker, M. L. , Deeter, L. , Goodrich, R. , Johnson, E. , Munday, D. , Mueller, R. , Kunz, D. , Jones, D. , Reese, V. , Summers, S. A. , Babu, P. V. A. , Holland, W. L. , Zhang, Q.‐J. , … Symons, J. D. (2015). Ceramide‐initiated protein phosphatase 2A activation contributes to arterial dysfunction in vivo. Diabetes, 64(11), 3914–3926. 10.2337/db15-0244 - DOI - PMC - PubMed
-
- Boyle, A. J. , Shih, H. , Hwang, J. , Ye, J. , Lee, B. , Zhang, Y. , Kwon, D. , Jun, K. , Zheng, D. , Sievers, R. , Angeli, F. , Yeghiazarians, Y. , Lee, R. (2011). Cardiomyopathy of aging in the mammalian heart is characterized by myocardial hypertrophy, fibrosis and a predisposition towards cardiomyocyte apoptosis and autophagy. Experimental Gerontology, 46(7), 549–559. S0531‐5565(11)00060‐X [pii]; 10.1016/j.exger.2011.02.010 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

