Light-Induced Modulation of Chiral Functions in G-Quadruplex-Photochrome Systems
- PMID: 34554762
- PMCID: PMC8503878
- DOI: 10.1021/acs.jpclett.1c02207
Light-Induced Modulation of Chiral Functions in G-Quadruplex-Photochrome Systems
Abstract
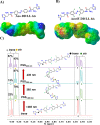
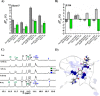
The design of artificially engineered chiral structures has received much attention, but the implementation of dynamic functions to modulate the chiroptical response of the systems is less explored. Here, we present a light-responsive G-quadruplex (G4)-based assembly in which chirality enrichment is induced, tuned, and fueled by molecular switches. In particular, the mirror-image dependence on photoactivated azo molecules, undergoing trans-to-cis isomerization, shows chiral recognition effects on the inherent flexibility and conformational diversity of DNA G4s having distinct handedness (right- and left-handed). Through a detailed experimental and computational analysis, we bring compelling evidence on the binding mode of the photochromes on G4s, and we rationalize the origin of the chirality effect that is associated with the complexation event.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

