In vitro effect of ferrous sulphate on bovine spermatozoa motility parameters, viability and Annexin V-labeled membrane changes
- PMID: 34555113
- PMCID: PMC8460022
- DOI: 10.1371/journal.pone.0257766
In vitro effect of ferrous sulphate on bovine spermatozoa motility parameters, viability and Annexin V-labeled membrane changes
Abstract
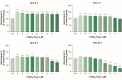
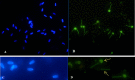
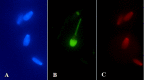
The aim of this study was to assess the dose- and time-dependent in vitro effects of ferrous sulphate (FeSO4.7H2O) on the motility parameters, viability, structural and functional activity of bovine spermatozoa. Spermatozoa motility parameters were determined after exposure to concentrations (3.90, 7.80, 15.60, 31.20, 62.50, 125, 250, 500 and 1000 μM) of FeSO4.7H2O using the SpermVisionTM CASA (Computer Assisted Semen Analyzer) system in different time periods. Cell viability was assessed by 3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide (MTT) assay, and the Annexin V-Fluos was applied to detect the membrane integrity of spermatozoa. The initial spermatozoa motility showed increased average values at all experimental concentrations compared to the control group (culture medium without FeSO4.7H2O). After 2 h, FeSO4.7H2O stimulated the overall percentage of spermatozoa motility at the concentrations of ≤ 125 μM. However, experimental administration of 250 μM of FeSO4.7H2O significantly (P < 0.001) decreased the spermatozoa motility but had no negative effect on the cell viability (P < 0.05) (Time 2 h). The lowest viability was noted after the addition of ≥ 500 μM of FeSO4.7H2O (P < 0.001). The concentrations of ≤ 62.50 μM of FeSO4.7H2O markedly stimulated (P < 0.001) spermatozoa activity after 24 h of exposure, while at high concentrations of ≥ 500 μM of FeSO4.7H2O the overall percentage of spermatozoa motility was significantly inhibited (P < 0.001) and it elicited cytotoxic action. Fluorescence analysis confirmed that spermatozoa incubated with higher concentrations (≥ 500 μM) of FeSO4.7H2O displayed apoptotic changes, as detected in head membrane (acrosomal part) and mitochondrial portion of spermatozoa. Moreover, the highest concentration and the longest time of exposure (1000 μM of FeSO4.7H2O; Time 6 h) induced even necrotic alterations to spermatozoa. These results suggest that high concentrations of FeSO4.7H2O are able to induce toxic effects on the structure and function of spermatozoa, while low concentrations may have the positive effect on the fertilization potential of spermatozoa.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Fergusson JE. The Heavy Elements: Chemistry, Environmental Impact and Health Effects. Oxford: Pergamon Press; 1990.
-
- Marzec-Wróblewska U, Kamiński P, Łakota P. Influence of chemical elements on mammalian spermatozoa. Folia Biol. 2012; 58: 7–15. - PubMed
-
- Kabata-Pendias A, Mukherjee AB. Trace Elements from Soil to Human, 1. ed., Germany Heidelberg: Springer; 2007.
-
- Eidi M, Eidi A, Pouyan O, Shahmohammadi P, Fazaeli R, Bahar M. Seminal plasma levels of copper and its relationship with seminal parameters. Iran J Reprod Med. 2010; 8: 60–65.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

