An optimized stepwise algorithm combining rapid antigen and RT-qPCR for screening of COVID-19 patients
- PMID: 34555117
- PMCID: PMC8460002
- DOI: 10.1371/journal.pone.0257817
An optimized stepwise algorithm combining rapid antigen and RT-qPCR for screening of COVID-19 patients
Abstract
Background & aim: We investigated the combination of rapid antigen detection (RAD) and RT-qPCR assays in a stepwise procedure to optimize the detection of COVID-19.
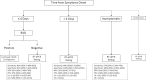
Methods: From August 2020 to November 2020, 43,399 patients were screened in our laboratory for COVID-19 diagnostic by RT-qPCR using nasopharyngeal swab. Overall, 4,691 of the 43,399 were found to be positive, and 200 were retrieved for RAD testing allowing comparison of diagnostic accuracy between RAD and RT-qPCR. Cycle threshold (Ct) and time from symptoms onset (TSO) were included as covariates.
Results: The overall sensitivity, specificity, PPV, NPV, LR-, and LR+ of RAD compared with RT-qPCR were 72% (95%CI 62%-81%), 99% (95% CI95%-100%), 99% (95%CI 93%-100%), and 78% (95%CI 70%-85%), 0.28 (95%CI 0.21-0.39), and 72 (95%CI 10-208) respectively. Sensitivity was higher for patients with Ct ≤ 25 regardless of TSO: TSO ≤ 4 days 92% (95%CI 75%-99%), TSO > 4 days 100% (95%CI 54%-100%), and asymptomatic 100% (95%CI 78-100%). Overall, combining RAD and RT-qPCR would allow reducing from only 4% the number of RT-qPCR needed.
Conclusions: This study highlights the risk of misdiagnosing COVID-19 in 28% of patients if RAD is used alone. A stepwise analysis that combines RAD and RT-qPCR would be an efficient screening procedure for COVID-19 detection and may facilitate the control of the outbreak.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Jayamohan H, Lambert CJ, Sant HJ, Jafek A, Patel D, Feng H, et al.. SARS-CoV-2 pandemic: a review of molecular diagnostic tools including sample collection and commercial response with associated advantages and limitations. Anal Bioanal Chem. 2020. doi: 10.1007/s00216-020-02958-1 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

