Phase 2 Randomized Study of Oral Ibrexafungerp Versus Fluconazole in Vulvovaginal Candidiasis
- PMID: 34555149
- PMCID: PMC9258939
- DOI: 10.1093/cid/ciab841
Phase 2 Randomized Study of Oral Ibrexafungerp Versus Fluconazole in Vulvovaginal Candidiasis
Abstract
Background: Vulvovaginal candidiasis affects approximately 75% of women in their lifetime. Approved treatment options are limited to oral or topical azoles. Ibrexafungerp, a novel, first-in-class oral triterpenoid glucan synthase inhibitor, has demonstrated broad fungicidal Candida activity and a favorable tolerability profile. The primary objective of this dose-finding study was to identify the optimal dose of oral ibrexafungerp in patients with acute vulvovaginal candidiasis.
Methods: Patients with vulvovaginal signs and symptoms score ≥7 were randomized equally to 6 treatments groups: 5 treatment doses of oral ibrexafungerp or oral fluconazole 150 mg. The primary endpoint was the percentage of patients with a clinical cure (complete resolution of vulvovaginal signs and symptoms) at the test-of-cure visit (day 10).
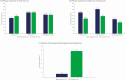
Results: Overall, 186 patients were randomized into the 6 treatment groups. Results, using the modified intent-to-treat population (baseline positive culture), are reported for ibrexafungerp 300 mg twice daily (BID) for 1 day (n = 27), which was the dose selected for phase 3 studies, and fluconazole 150 mg for 1 day (n = 24). At day 10, the clinical cure rates for ibrexafungerp and fluconazole were 51.9% and 58.3%, respectively; at day 25, patients with no signs or symptoms were 70.4% and 50.0%, respectively. During the study ibrexafungerp patients required less antifungal rescue medications compared with fluconazole (3.7% vs 29.2%, respectively). Ibrexafungerp was well tolerated, with the most common treatment-related adverse events being mild gastrointestinal events.
Conclusions: Ibrexafungerp is a well-tolerated novel antifungal with comparable efficacy to fluconazole in the treatment of acute vulvovaginal candidiasis.
Clinical trials registration: NCT03253094.
Keywords: Candida albicans; SCY-078; fluconazole; ibrexafungerp; vulvovaginal candidiasis.
© The Author(s) 2021. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures
References
-
- Mendling W, Brasch J, Cornely OA, et al. . Guideline: vulvovaginal candidosis (AWMF 015/072), S2k (excluding chronic mucocutaneous candidosis). Mycoses 2015; 58:1–15. - PubMed
-
- Jeanmonod R, Jeanmonod D.. Vaginal candidiasis. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing, 2020. Available at: https://www.ncbi.nlm.nih.gov/books/NBK459317/. Updated 21 November 2020. Accessed 9 December 2020.
-
- Committee on Practice Bulletins—Gynecology. Vaginitis in nonpregnant patients: ACOG practice bulletin, Number 215. Obstet Gynecol 2020; 135:e1–17. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

