Added value of rapid respiratory syndromic testing at point of care versus central laboratory testing: a controlled clinical trial
- PMID: 34555158
- PMCID: PMC8460108
- DOI: 10.1093/jac/dkab241
Added value of rapid respiratory syndromic testing at point of care versus central laboratory testing: a controlled clinical trial
Abstract
Background: Virus-associated respiratory infections are in the spotlight with the emergence of SARS-CoV-2 and the expanding use of multiplex PCR (mPCR). The impact of molecular testing as a point-of-care test (POCT) in the emergency department (ED) is still unclear.
Objectives: To compare the impact of a syndromic test performed in the ED as a POCT and in the central laboratory on length of stay (LOS), antibiotic use and single-room assignment.
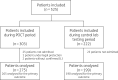
Methods: From 19 November 2019 to 9 March 2020, adults with acute respiratory illness seeking care in the ED of a large hospital were enrolled, with mPCR performed with a weekly alternation in the ED as a POCT (week A) or in the central laboratory (week B).
Results: 474 patients were analysed: 275 during A weeks and 199 during B weeks. Patient characteristics were similar. The hospital LOS (median 7 days during week A versus 7 days during week B, P = 0.29), the proportion of patients with ED-LOS <1 day (63% versus 60%, P = 0.57) and ED antibiotic prescription (59% versus 58%, P = 0.92) were not significantly different. Patients in the POCT arm were more frequently assigned a single room when having a positive PCR for influenza, respiratory syncytial virus and metapneumovirus [52/70 (74%) versus 19/38 (50%) in the central testing arm, P = 0.012].
Conclusions: Syndromic testing performed in the ED compared with the central laboratory failed to reduce the LOS or antibiotic consumption in patients with acute respiratory illness, but was associated with an increased single-room assignment among patients in whom a significant respiratory pathogen was detected.
© The Author(s) 2021. Published by Oxford University Press on behalf of the British Society for Antimicrobial Chemotherapy.
Figures
Similar articles
-
Impact of Fast SARS-CoV-2 Molecular Point-Of-Care Testing on Patients' Length of Stay in an Emergency Department.Microbiol Spectr. 2022 Aug 31;10(4):e0063622. doi: 10.1128/spectrum.00636-22. Epub 2022 Jun 22. Microbiol Spectr. 2022. PMID: 35730967 Free PMC article.
-
Use of point-of-care testing and early assessment model reduces length of stay for ambulatory patients in an emergency department.Scand J Trauma Resusc Emerg Med. 2016 Oct 18;24(1):125. doi: 10.1186/s13049-016-0319-z. Scand J Trauma Resusc Emerg Med. 2016. PMID: 27756354 Free PMC article.
-
Molecular point-of-care testing for respiratory viruses versus routine clinical care in adults with acute respiratory illness presenting to secondary care: a pragmatic randomised controlled trial protocol (ResPOC).BMC Infect Dis. 2017 Feb 6;17(1):128. doi: 10.1186/s12879-017-2219-x. BMC Infect Dis. 2017. PMID: 28166743 Free PMC article.
-
Syndromic and Point-of-Care Molecular Testing.Clin Lab Med. 2022 Dec;42(4):507-531. doi: 10.1016/j.cll.2022.09.008. Clin Lab Med. 2022. PMID: 36368779 Review. No abstract available.
-
Multiplex Molecular Point-of-Care Test for Syndromic Infectious Diseases.Biochip J. 2021;15(1):14-22. doi: 10.1007/s13206-021-00004-5. Epub 2021 Feb 15. Biochip J. 2021. PMID: 33613852 Free PMC article. Review.
Cited by
-
The QuantuMDx Q-POC SARS-CoV-2 RT-PCR assay for rapid detection of COVID-19 at point-of-care: preliminary evaluation of a novel technology.Sci Rep. 2023 Jun 17;13(1):9827. doi: 10.1038/s41598-023-35479-9. Sci Rep. 2023. PMID: 37330592 Free PMC article.
-
A pragmatic randomized controlled trial of rapid on-site influenza and respiratory syncytial virus PCR testing in paediatric and adult populations.BMC Infect Dis. 2022 Nov 16;22(1):854. doi: 10.1186/s12879-022-07796-3. BMC Infect Dis. 2022. PMID: 36384484 Free PMC article. Clinical Trial.
-
Diagnostic Stewardship in Community-Acquired Pneumonia With Syndromic Molecular Testing: A Randomized Clinical Trial.JAMA Netw Open. 2024 Mar 4;7(3):e240830. doi: 10.1001/jamanetworkopen.2024.0830. JAMA Netw Open. 2024. PMID: 38446481 Free PMC article. Clinical Trial.
-
Associations between hospital structure, infection control and incidence of hospital-acquired viral respiratory infections: a 10-year surveillance study.Antimicrob Resist Infect Control. 2025 Apr 11;14(1):28. doi: 10.1186/s13756-025-01543-4. Antimicrob Resist Infect Control. 2025. PMID: 40217353 Free PMC article.
-
Syndromic testing for the diagnosis of infectious diseases: the right test if used for the right patient.J Antimicrob Chemother. 2021 Sep 23;76(Suppl 3):iii2-iii3. doi: 10.1093/jac/dkab248. J Antimicrob Chemother. 2021. PMID: 34555153 Free PMC article. No abstract available.
References
-
- Legand A, Briand S, Shindo N. et al.Addressing the public health burden of respiratory viruses: the Battle against Respiratory Viruses (BRaVe) initiative. Future Virol 2013; 8: 953–68.
-
- van Asten L, van den Wijngaard C, van Pelt W. et al.Mortality attributable to 9 common infections: significant effect of influenza A, respiratory syncytial virus, influenza B, norovirus, and parainfluenza in elderly persons. J Infect Dis 2012; 206: 628–39. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous