Architecture and assembly mechanism of native glycine receptors
- PMID: 34555840
- PMCID: PMC8647860
- DOI: 10.1038/s41586-021-04022-z
Architecture and assembly mechanism of native glycine receptors
Abstract
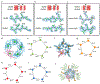
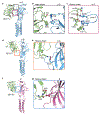
Glycine receptors (GlyRs) are pentameric, 'Cys-loop' receptors that form chloride-permeable channels and mediate fast inhibitory signalling throughout the central nervous system1,2. In the spinal cord and brainstem, GlyRs regulate locomotion and cause movement disorders when mutated2,3. However, the stoichiometry of native GlyRs and the mechanism by which they are assembled remain unclear, despite extensive investigation4-8. Here we report cryo-electron microscopy structures of native GlyRs from pig spinal cord and brainstem, revealing structural insights into heteromeric receptors and their predominant subunit stoichiometry of 4α:1β. Within the heteromeric pentamer, the β(+)-α(-) interface adopts a structure that is distinct from the α(+)-α(-) and α(+)-β(-) interfaces. Furthermore, the β-subunit contains a unique phenylalanine residue that resides within the pore and disrupts the canonical picrotoxin site. These results explain why inclusion of the β-subunit breaks receptor symmetry and alters ion channel pharmacology. We also find incomplete receptor complexes and, by elucidating their structures, reveal the architectures of partially assembled α-trimers and α-tetramers.
© 2021. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Competing Interests
The authors declare no competing interests.
Figures


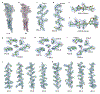






References
Main References
-
- Lynch JW Molecular structure and function of the glycine receptor chloride channel. Physiol. Rev 84, 1051–1095 (2004). - PubMed
-
- Shiang R et al. Mutations in the alpha 1 subunit of the inhibitory glycine receptor cause the dominant neurologic disorder, hyperekplexia. Nat. Gene.t 5, 351–358 (1993). - PubMed
-
- Grudzinska J et al. The beta subunit determines the ligand binding properties of synaptic glycine receptors. Neuron 45, 727–739 (2005). - PubMed
Method References
-
- Kawate T & Gouaux E Fluorescence-detection size-exclusion chromatography for precrystallization screening of integral membrane proteins. Structure 14, 673–681 (2006). - PubMed
-
- Cook N, Harris A, Hopkins A & Hughes K Scintillation proximity assay (SPA) technology to study biomolecular interactions. Curr. Protoc. Protein Sci. Chapter 19, Unit 19.8 (2002). - PubMed
-
- Cheng Y & Prusoff WH Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem. Pharmacol 22, 3099–3108 (1973). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

