Seroprevalence of SARS-CoV-2-specific IgG antibodies in Kashmir, India, 7 months after the first reported local COVID-19 case: results of a population-based seroprevalence survey from October to November 2020
- PMID: 34556519
- PMCID: PMC8461364
- DOI: 10.1136/bmjopen-2021-053791
Seroprevalence of SARS-CoV-2-specific IgG antibodies in Kashmir, India, 7 months after the first reported local COVID-19 case: results of a population-based seroprevalence survey from October to November 2020
Abstract
Objectives: We designed a population-based survey in Kashmir to estimate the seroprevalence of SARS-CoV-2-specific IgG antibodies in the general population aged 18 years and above.
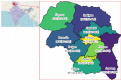
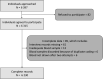
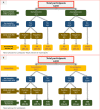
Setting: The survey was conducted among 110 villages and urban wards across 10 districts in Kashmir from 17 October 2020 to 4 November 2020.
Participants: Individuals aged 18 years and above were eligible to be included in the survey. Serum samples were tested for the presence of SARS-CoV-2-specific IgG antibodies using the Abbott SARS-CoV-2 IgG assay.
Primary and secondary outcome measures: We labelled assay results equal to or above the cut-off index value of 1.4 as positive for SARS-CoV-2-specific IgG antibodies. Seroprevalence estimates were adjusted for the sampling design and assay characteristics.
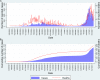
Results: Out of 6397 eligible individuals enumerated, 6315 (98.7%) agreed to participate. The final analysis was done on 6230 participants. Seroprevalence adjusted for the sampling design and assay characteristics was 36.7% (95% CI 34.3% to 39.2%). Seroprevalence was higher among the older population. Among seropositive individuals, 10.2% (247/2415) reported a history of COVID-19-like symptoms. Out of 474 symptomatic individuals, 233 (49.2%) reported having been tested. We estimated an infection fatality rate of 0.034%.
Conclusions: During the first 7 months of the COVID-19 epidemic in Kashmir Valley, approximately 37% of individuals were infected. The reported number of COVID-19 cases was only a small fraction of the estimated number of infections. A more efficient surveillance system with strengthened reporting of COVID-19 cases and deaths is warranted.
Keywords: COVID-19; epidemiology.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- Timeline of WHO’s response to COVID-19. Available: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/interact... [Accessed 26 Mar 2021].
-
- Saleem S, Quansar R, Qurieshi M. COVID-19: preparedness and response by Union territory of Jammu and Kashmir for containment of pandemic. Curr Med Issues 2020;18:206. 10.4103/cmi.cmi_56_20 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
