Self-management intervention to reduce pulmonary exacerbations by supporting treatment adherence in adults with cystic fibrosis: a randomised controlled trial
- PMID: 34556552
- PMCID: PMC9016257
- DOI: 10.1136/thoraxjnl-2021-217594
Self-management intervention to reduce pulmonary exacerbations by supporting treatment adherence in adults with cystic fibrosis: a randomised controlled trial
Abstract
Introduction: Recurrent pulmonary exacerbations lead to progressive lung damage in cystic fibrosis (CF). Inhaled medications (mucoactive agents and antibiotics) help prevent exacerbations, but objectively measured adherence is low. We investigated whether a multi-component (complex) self-management intervention to support adherence would reduce exacerbation rates over 12 months.
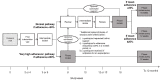
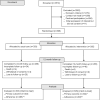
Methods: Between October 2017 and May 2018, adults with CF (aged ≥16 years; 19 UK centres) were randomised to the intervention (data-logging nebulisers, a digital platform and behavioural change sessions with trained clinical interventionists) or usual care (data-logging nebulisers). Outcomes included pulmonary exacerbations (primary outcome), objectively measured adherence, body mass index (BMI), lung function (FEV1) and Cystic Fibrosis Questionnaire-Revised (CFQ-R). Analyses were by intent to treat over 12 months.
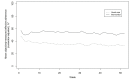
Results: Among intervention (n=304) and usual care (n=303) participants (51% female, median age 31 years), 88% completed 12-month follow-up. Mean exacerbation rate was 1.63/year with intervention and 1.77/year with usual care (adjusted ratio 0.96; 95% CI 0.83 to 1.12; p=0.64). Adjusted mean differences (95% CI) were in favour of the intervention versus usual care for objectively measured adherence (9.5% (8.6% to 10.4%)) and BMI (0.3 (0.1 to 0.6) kg/m2), with no difference for %FEV1 (1.4 (-0.2 to 3.0)). Seven CFQ-R subscales showed no between-group difference, but treatment burden reduced for the intervention (3.9 (1.2 to 6.7) points). No intervention-related serious adverse events occurred.
Conclusions: While pulmonary exacerbations and FEV1 did not show statistically significant differences, the intervention achieved higher objectively measured adherence versus usual care. The adherence difference might be inadequate to influence exacerbations, though higher BMI and lower perceived CF treatment burden were observed.
Keywords: cystic fibrosis; nebuliser therapy; psychology.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: no direct influence of any competing interest on the submitted work; support for MJW from Philips Respironics for early data transfer experience; support for the University of Manchester software team from PARI Pharma to create a medication reporting component within the CFHealthHub software; funding for MJW from Zambon; funding for IB from Microsoft Research; SJW is an NIHR Senior Investigator; IB became Chief Data Scientist (advisory) for AstraZeneca in September 2019; no other financial relationships that might have an interest in the submitted work in the previous three years; and no other relationships or activities that could appear to have influenced the submitted work.
Figures
Comment in
-
Adherence to CF treatment can be improved with the right approach!Thorax. 2022 May;77(5):428. doi: 10.1136/thoraxjnl-2021-218134. Epub 2021 Oct 22. Thorax. 2022. PMID: 34686569 No abstract available.
References
-
- World Health Organization . Adherence to long-term therapies. Evidence for action, 2003. Available: https://www.who.int/chp/knowledge/publications/adherence_report/en/
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
