CSF Synaptic Biomarkers in the Preclinical Stage of Alzheimer Disease and Their Association With MRI and PET: A Cross-sectional Study
- PMID: 34556565
- PMCID: PMC8610620
- DOI: 10.1212/WNL.0000000000012853
CSF Synaptic Biomarkers in the Preclinical Stage of Alzheimer Disease and Their Association With MRI and PET: A Cross-sectional Study
Abstract
Background and objectives: To determine whether CSF synaptic biomarkers are altered in the early preclinical stage of the Alzheimer continuum and associated with Alzheimer disease (AD) risk factors, primary pathology, and neurodegeneration markers.
Methods: This cross-sectional study was performed in the Alzheimer's and Families (ALFA+) cohort, comprising middle-aged cognitively unimpaired participants. CSF neurogranin and growth-associated protein-43 (GAP-43) were measured with immunoassays, and synaptosomal-associated protein-25 (SNAP-25) and synaptotagmin-1 were measured with immunoprecipitation mass spectrometry. AD CSF biomarkers β-amyloid (Aβ)42/40, phosphorylated tau (p-tau), and total tau and the neurodegeneration biomarker neurofilament light chain (NfL) were also measured. Participants underwent structural MRI and fluorodeoxyglucose and Aβ PET imaging. General linear modeling was used to test the associations between CSF synaptic biomarkers and risk factors, Aβ pathology, tau pathology, and neurodegeneration markers.
Results: All CSF synaptic biomarkers increased with age. CSF neurogranin was higher in females, while CSF SNAP-25 was higher in APOE ε4 carriers. All CSF synaptic biomarkers increased with higher Aβ load (as measured by CSF Aβ42/40 and Aβ PET Centiloid values), and it is important to note that the synaptic biomarkers were increased even in individuals in the earliest stages of Aβ deposition. Higher CSF synaptic biomarkers were also associated with higher CSF p-tau and NfL. Higher CSF neurogranin and GAP-43 were significantly associated with higher brain metabolism but lower cortical thickness in AD-related brain regions.
Discussion: CSF synaptic biomarkers increase in the early preclinical stages of the Alzheimer continuum even when a low burden of Aβ pathology is present, and they differ in their association with age, sex, APOE ε4, and markers of neurodegeneration.
Trial registration information: ClinicalTrials.gov Identifier NCT02485730.
Copyright © 2021 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Figures
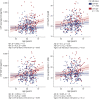
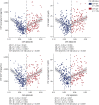


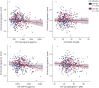
References
-
- Selkoe DJ. Alzheimer's disease is a synaptic failure. Science. 2002;298(5594):789-791. - PubMed
-
- Arendt T. Synaptic degeneration in Alzheimer's disease. Acta Neuropathol. 2009;118(1):167-179. - PubMed
-
- DeKosky ST, Scheff SW. Synapse loss in frontal cortex biopsies in Alzheimer's disease: correlation with cognitive severity. Ann Neurol. 1990;27(5):457-464. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
