Scene statistics and noise determine the relative arrangement of receptive field mosaics
- PMID: 34556573
- PMCID: PMC8488585
- DOI: 10.1073/pnas.2105115118
Scene statistics and noise determine the relative arrangement of receptive field mosaics
Abstract
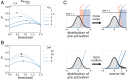
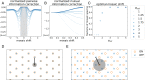
Many sensory systems utilize parallel ON and OFF pathways that signal stimulus increments and decrements, respectively. These pathways consist of ensembles or grids of ON and OFF detectors spanning sensory space. Yet, encoding by opponent pathways raises a question: How should grids of ON and OFF detectors be arranged to optimally encode natural stimuli? We investigated this question using a model of the retina guided by efficient coding theory. Specifically, we optimized spatial receptive fields and contrast response functions to encode natural images given noise and constrained firing rates. We find that the optimal arrangement of ON and OFF receptive fields exhibits a transition between aligned and antialigned grids. The preferred phase depends on detector noise and the statistical structure of the natural stimuli. These results reveal that noise and stimulus statistics produce qualitative shifts in neural coding strategies and provide theoretical predictions for the configuration of opponent pathways in the nervous system.
Keywords: computational model; efficient coding; mosaic; retina.
Copyright © 2021 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures




References
-
- Joesch M., Schnell B., Raghu S. V., Reiff D. F., Borst A., On and off pathways in drosophila motion vision. Nature 468, 300–304 (2010). - PubMed
-
- Kuffler S. W., Discharge patterns and functional organization of mammalian retina. J. Neurophysiol. 16, 37–68 (1953). - PubMed
-
- Smith E. C., Lewicki M. S., Efficient auditory coding. Nature 439, 978–982 (2006). - PubMed
-
- Chalasani S. H., et al. ., Dissecting a circuit for olfactory behaviour in caenorhabditis elegans. Nature 450, 63–70 (2007). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

