Liver Targeting of Daclatasvir via Tailoring Sterically Stabilized Bilosomes: Fabrication, Comparative In Vitro/In Vivo Appraisal and Biodistribution Studies
- PMID: 34556987
- PMCID: PMC8455511
- DOI: 10.2147/IJN.S319255
Liver Targeting of Daclatasvir via Tailoring Sterically Stabilized Bilosomes: Fabrication, Comparative In Vitro/In Vivo Appraisal and Biodistribution Studies
Abstract
Introduction: Hepatitis C virus (HCV) is a significant public health concern that threatens millions of individuals worldwide. Daclatasvir (DAC) is a promising direct-acting antiviral approved for treating HCV infection around the world. The goal of this study was to encapsulate DAC into novel polyethylene glycol (PEG) decorated bilosomes (PEG-BILS) to achieve enhanced drug delivery to the liver.
Methods: DAC-loaded BILS were primed by a thin film hydrating technique. The study of the impact of various formulation variables on the properties of BILS and selection of the optimal formulation was generated using Design-Expert® software. The optimum preparation was then pegylated via the incorporation of PEG-6-stearate (5% w/w, with respect to the lipid phase).
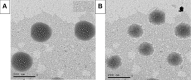
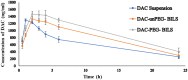
Results: The optimum PEG-BILS formulation, containing PL:SDC ratio (5:1), 5 mg cholesterol, and 30 min sonication, yielded spherical vesicles in the nanoscale (200±15.2 nm), elevated percent of entrapment efficiency (95.5±7.77%), and a sustained release profile of DAC with 35.11±2.3% release. In vivo and drug distribution studies revealed an enhanced hepatocellular delivery of DAC-loaded PEG-BILS compared to DAC-unPEG-BILS and DAC suspension, where DAC-PEG-BILS achieved 1.19- and 1.54 times the AUC0-24 of DAC-unPEG-BILS and DAC suspension, respectively. Compared with DAC-unPEG-BILS and DAC suspension, DAC-PEG-BILS delivered about 2 and 3 times higher DAC into the liver, respectively.
Conclusion: The innovative encapsulation of DAC-PEG-BILS has a great potential for liver targeting.
Keywords: Box–Behnken approach; antiviral drug; bile-based nanovesicles; bioavailability; liver targeting parameters; nanodrug delivery system; pharmacokinetic.
© 2021 El-Nabarawi et al.
Conflict of interest statement
The authors state no conflicts of interest. The content and the writing of this article are the responsibility of the authors only.
Figures

References
-
- Zaman B, Siddique F, Hassan W. RP-HPLC method for simultaneous determination of sofosbuvir and ledipasvir in tablet dosage form and its application to in vitro dissolution studies. Chromatographia. 2016;79(23–24):1605–1613. doi:10.1007/s10337-016-3179-9 - DOI
-
- Jagadabi V, Nagendra Kumar P, Mahesh K, Pamidi S, Ramaprasad L, Nagaraju D. A stability-indicating UPLC method for the determination of potential impurities and its mass by a new QDa mass detector in daclatasvir drug used to treat hepatitis C infection. J Chromatogr Sci. 2019;57(1):44–53. doi:10.1093/chromsci/bmy079 - DOI - PubMed
-
- Böttger R, Pauli G, Chao P-H, Al-Fayez N, Hohenwarter L, Li S-D. Lipid-based nanoparticle technologies for liver targeting. Adv Drug Deliv Rev. 2020;154–155:79–101. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

