Distribution of Aldh1L1-CreERT2 Recombination in Astrocytes Versus Neural Stem Cells in the Neurogenic Niches of the Adult Mouse Brain
- PMID: 34557065
- PMCID: PMC8452868
- DOI: 10.3389/fnins.2021.713077
Distribution of Aldh1L1-CreERT2 Recombination in Astrocytes Versus Neural Stem Cells in the Neurogenic Niches of the Adult Mouse Brain
Abstract
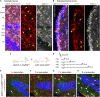
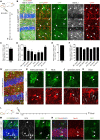
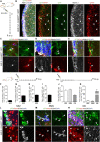
In the adult central nervous system, neural stem cells (NSCs) reside in two discrete niches: the subependymal zone (SEZ) of the lateral ventricle and the subgranular zone (SGZ) of the dentate gyrus (DG). Here, NSCs represent a population of highly specialized astrocytes that are able to proliferate and give rise to neuronal and glial progeny. This process, termed adult neurogenesis, is extrinsically regulated by other niche cells such as non-stem cell astrocytes. Studying these non-stem cell niche astrocytes and their role during adult neuro- and gliogenesis has been hampered by the lack of genetic tools to discriminate between transcriptionally similar NSCs and niche astrocytes. Recently, Aldh1L1 has been shown to be a pan-astrocyte marker and that its promoter can be used to specifically target astrocytes using the Cre-loxP system. In this study we explored whether the recently described Aldh1L1-CreERT2 mouse line (Winchenbach et al., 2016) can serve to specifically target niche astrocytes without inducing recombination in NSCs in adult neurogenic niches. Using short- and long-term tamoxifen protocols we revealed high recombination efficiency and specificity in non-stem cell astrocytes and little to no recombination in NSCs of the adult DG. However, in the SEZ we observed recombination in ependymal cells, astrocytes, and NSCs, the latter giving rise to neuronal progeny of the rostral migratory stream and olfactory bulb. Thus, we recommend the here described Aldh1L1-CreERT2 mouse line for predominantly studying the functions of non-stem cell astrocytes in the DG under physiological and pathological conditions.
Keywords: Aldh1L1; Aldh1L1-CreERT2; astrocytes; dentate gyrus; neural stem cells; neurogenic niche; subependymal zone.
Copyright © 2021 Beyer, Lüdje, Karpf, Saher and Beckervordersandforth.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

