Characterizing the Retinal Phenotype of the Thy1-h[A30P]α-syn Mouse Model of Parkinson's Disease
- PMID: 34557068
- PMCID: PMC8452874
- DOI: 10.3389/fnins.2021.726476
Characterizing the Retinal Phenotype of the Thy1-h[A30P]α-syn Mouse Model of Parkinson's Disease
Abstract
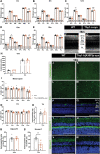
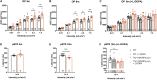
Despite decades of research, disease-modifying treatments of Parkinson's disease (PD), the second most common neurodegenerative disease worldwide, remain out of reach. One of the reasons for this treatment gap is the incomplete understanding of how misfolded alpha-synuclein (α-syn) contributes to PD pathology. The retina, as an integral part of the central nervous system, recapitulates the PD disease processes that are typically seen in the brain, and retinal manifestations have emerged as prodromal symptoms of the disease. The timeline of PD manifestations in the visual system, however, is not fully elucidated and the underlying mechanisms are obscure. This highlights the need for new studies investigating retinal pathology, in order to propel its use as PD biomarker, and to develop validated research models to investigate PD pathogenesis. The present study pioneers in characterizing the retina of the Thy1-h[A30P]α-syn PD transgenic mouse model. We demonstrate widespread α-syn accumulation in the inner retina of these mice, of which a proportion is phosphorylated yet not aggregated. This α-syn expression coincides with inner retinal atrophy due to postsynaptic degeneration. We also reveal abnormal retinal electrophysiological responses. Absence of selective loss of melanopsin retinal ganglion cells or dopaminergic amacrine cells and inflammation indicates that the retinal manifestations in these transgenic mice diverge from their brain phenotype, and questions the specific cellular or molecular alterations that underlie retinal pathology in this PD mouse model. Nevertheless, the observed α-syn accumulation, synapse loss and functional deficits suggest that the Thy1-h[A30P]α-syn retina mimics some of the features of prodromal PD, and thus may provide a window to monitor and study the preclinical/prodromal stages of PD, PD-associated retinal disease processes, as well as aid in retinal biomarker discovery and validation.
Keywords: Parkinson’s disease; alpha-synuclein; retina; transgenic mouse model; visual system.
Copyright © 2021 Veys, Devroye, Lefevere, Cools, Vandenabeele and De Groef.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

