Gut Microbiota Modulation as a Potential Target for the Treatment of Lung Infections
- PMID: 34557097
- PMCID: PMC8453009
- DOI: 10.3389/fphar.2021.724033
Gut Microbiota Modulation as a Potential Target for the Treatment of Lung Infections
Abstract
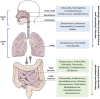
The gastrointestinal and respiratory systems are colonized by a complex ecosystem of microorganisms called the microbiota. These microorganisms co-evolved over millions of years with the host, creating a symbiotic relationship that is fundamental for promoting host homeostasis by producing bioactive metabolites and antimicrobial molecules, and regulating the immune and inflammatory responses. Imbalance in the abundance, diversity, and function of the gut microbiota (known as dysbiosis) have been shown to increase host susceptibility to infections in the lungs, suggesting crosstalk between these organs. This crosstalk is now referred to as the gut-lung axis. Hence, the use of probiotics, prebiotics, and synbiotics for modulation of gut microbiota has been studied based on their effectiveness in reducing the duration and severity of respiratory tract infections, mainly owing to their effects on preventing pathogen colonization and modulating the immune system. This review discusses the role and responses of probiotics, prebiotics, and synbiotics in the gut-lung axis in the face of lung infections.
Keywords: gut-lung axis; immunobiotics; inflammation; microbiota; mucosal immmunity; prebiotcs; probiotics; symbiotics.
Copyright © 2021 Cruz, Ricci and Vieira.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Antunes K. H., Fachi J. L., de Paula R., da Silva E. F., Pral L. P., dos Santos A. Á., et al. (2019). Microbiota-Derived Acetate Protects Against Respiratory Syncytial Virus Infection Through a GPR43-Type 1 Interferon Response. Nat. Commun. 10, 3273–3317. 10.1038/s41467-019-11152-6 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

