Staphylococcus aureus Strain-Dependent Biofilm Formation in Bone-Like Environment
- PMID: 34557170
- PMCID: PMC8453086
- DOI: 10.3389/fmicb.2021.714994
Staphylococcus aureus Strain-Dependent Biofilm Formation in Bone-Like Environment
Abstract
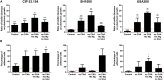
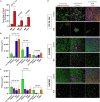
Staphylococcus aureus species is an important threat for hospital healthcare because of frequent colonization of indwelling medical devices such as bone and joint prostheses through biofilm formations, leading to therapeutic failure. Furthermore, bacteria within biofilm are less sensitive to the host immune system responses and to potential antibiotic treatments. We suggested that the periprosthetic bone environment is stressful for bacteria, influencing biofilm development. To provide insights into S. aureus biofilm properties of three strains [including one methicillin-resistant S. aureus (MRSA)] under this specific environment, we assessed several parameters related to bone conditions and expected to affect biofilm characteristics. We reported that the three strains harbored different behaviors in response to the lack of oxygen, casamino acids and glucose starvation, and high concentration of magnesium. Each strain presented different biofilm biomass and live adherent cells proportion, or matrix production and composition. However, the three strains shared common responses in a bone-like environment: a similar production of extracellular DNA and engagement of the SOS response. This study is a step toward a better understanding of periprosthetic joint infections and highlights targets, which could be common among S. aureus strains and for future antibiofilm strategies.
Keywords: MRSA; MSSA; biofilms; bone microenvironment; prosthetic joint infection.
Copyright © 2021 Lamret, Varin-Simon, Velard, Terryn, Mongaret, Colin, Gangloff and Reffuveille.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

