B-Cell Immunophenotyping to Predict Vaccination Outcome in the Immunocompromised - A Systematic Review
- PMID: 34557188
- PMCID: PMC8452967
- DOI: 10.3389/fimmu.2021.690328
B-Cell Immunophenotyping to Predict Vaccination Outcome in the Immunocompromised - A Systematic Review
Abstract
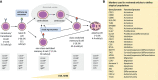
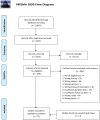
Vaccination is the most effective measure to prevent infections in the general population. Its efficiency strongly depends on the function and composition of the immune system. If the immune system lacks critical components, patients will not be fully protected despite a completed vaccination schedule. Antigen-specific serum immunoglobulin levels are broadly used correlates of protection. These are the products of terminally differentiated B cells - plasma cells. Here we reviewed the literature on how aberrancies in B-cell composition and function influence immune responses to vaccinations. In a search through five major literature databases, 6,537 unique articles published from 2000 and onwards were identified. 75 articles were included along three major research lines: extremities of life, immunodeficiency and immunosuppression. Details of the protocol can be found in the International Prospective Register of Systematic Reviews [PROSPERO (registration number CRD42021226683)]. The majority of articles investigated immune responses in adults, in which vaccinations against pneumococci and influenza were strongly represented. Lack of baseline information was the most common reason of exclusion. Irrespective of study group, three parameters measured at baseline seemed to have a predictive value in assessing vaccine efficacy: (1) distribution of B-cell subsets (mostly a reduction in memory B cells), (2) presence of exhausted/activated B cells, or B cells with an aberrant phenotype, and (3) pre-existing immunological memory. In this review we showed how pre-immunization (baseline) knowledge of circulating B cells can be used to predict vaccination efficacy. We hope that this overview will contribute to optimizing vaccination strategies, especially in immunocompromised patients.
Keywords: B cells; extremities of life; immune monitoring; immunodeficiency; immunosuppression; vaccination.
Copyright © 2021 Diks, Overduin, van Leenen, Slobbe, Jolink, Visser, van Dongen and Berkowska.
Conflict of interest statement
AD, JD, and MB report inventorship of the patent “Means and methods for multiparameter cytometry-based leukocyte subsetting” (NL2844751, filing date 5 November 2019), owned by the EuroFlow Consortium (134). In addition, JD reports to be chairman of the EuroFlow scientific foundation, which receives royalties from licensed patents, which are collectively owned by the participants of the EuroFlow Foundation. These royalties are exclusively used for continuation of the EuroFlow collaboration and sustainability of the EuroFlow consortium. Lastly, JD reports an Educational Services Agreement from BD Biosciences (San José, CA) and a Scientific Advisor Agreement with Cytognos, all related fees and honoraria go to LUMC. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- CDC . Vaccines and Preventable Diseases. (2016). Available at: https://www.cdc.gov/vaccines/vpd/index.html.
-
- Bennett NM, Whitney CG, Moore M, Pilishvili T, Dooling KL. Use of 13-Valent Pneumococcal Conjugate Vaccine and 23-Valent Pneumococcal Polysaccharide Vaccine for Adults With Immunocompromising Conditions: Recommendations of the Advisory Committee on Immunization Practices (ACIP). Am J Transplant (2012) 13(1):232–5. 10.1111/ajt.12073 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

