Refraining from use diminishes cannabis-associated epigenetic changes in human sperm
- PMID: 34557312
- PMCID: PMC8455898
- DOI: 10.1093/eep/dvab009
Refraining from use diminishes cannabis-associated epigenetic changes in human sperm
Abstract
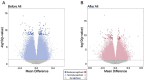
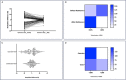
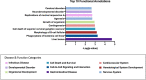
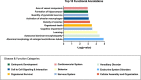
Cannabis use alters sperm DNA methylation, but the potential reversibility of these changes is unknown. Semen samples from cannabis users and non-user controls were collected at baseline and again following a 77-day period of cannabis abstinence (one spermatogenic cycle). Users and controls did not significantly differ by demographics or semen analyses. Whole-genome bisulfite sequencing identified 163 CpG sites with significantly different DNA methylation in sperm between groups (P < 2.94 × 10-9). Genes associated with altered CpG sites were enriched with those involved in development, including cardiogenesis and neurodevelopment. Many of the differences in sperm DNA methylation between groups were diminished after cannabis abstinence. These results indicate that sustained cannabis abstinence significantly reduces the number of sperm showing cannabis-associated alterations at genes important for early development.
Keywords: DNA methylation; cannabis; development; epigenetics; sperm.
© The Author(s) 2021. Published by Oxford University Press.
Figures

References
-
- Holloway ZR, Hawkey AB, Pippin E. et al.Paternal factors in neurodevelopmental toxicology: THC exposure of male rats causes long-lasting neurobehavioral effects in their offspring. Neurotoxicology 2020;78:57–63. - PubMed
-
- Levin ED, Hawkey AB, Hall BJ. et al.Paternal THC exposure in rats causes long-lasting neurobehavioral effects in the offspring. Neurotoxicol Teratol 2019;74:106806. - PubMed
-
- Slotkin TA, Skavicus S, Levin ED. et al.Paternal Δ9-tetrahydrocannabinol exposure prior to mating elicits deficits in cholinergic synaptic function in the offspring. Toxicol Sci 2020;174:210–7. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

