Cross-reactive Antibody Response to mRNA SARS-CoV-2 Vaccine After Recent COVID-19-Specific Monoclonal Antibody Therapy
- PMID: 34557558
- PMCID: PMC8454518
- DOI: 10.1093/ofid/ofab420
Cross-reactive Antibody Response to mRNA SARS-CoV-2 Vaccine After Recent COVID-19-Specific Monoclonal Antibody Therapy
Abstract
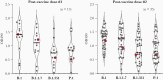
The efficacy of coronavirus disease 2019 (COVID-19) vaccines administered after COVID-19-specific monoclonal antibody is unknown, and "antibody interference" might hinder immune responses leading to vaccine failure. In an institutional review board-approved prospective study, we found that an individual who received mRNA COVID-19 vaccination <40 days after COVID-19-specific monoclonal antibody therapy for symptomatic COVID-19 had similar postvaccine antibody responses to SARS-CoV-2 receptor binding domain (RBD) for 4 important SARS-CoV-2 variants (B.1, B.1.1.7, B.1.351, and P.1) as other participants who were also vaccinated following COVID-19. Vaccination against COVID-19 shortly after COVID-19-specific monoclonal antibody can boost and expand antibody protection, questioning the need to delay vaccination in this setting.
Trial registration: The St. Jude Tracking of Viral and Host Factors Associated with COVID-19 study; NCT04362995; https://clinicaltrials.gov/ct2/show/NCT04362995.
Keywords: COVID-19; SARS-CoV-2; antibody; bamlanivimab; vaccine failure.
© The Author(s) 2021. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Figures

References
-
- Centers for Disease Control and Prevention. Interim clinical considerations for use of COVID-19 vaccines currently authorized in the United States. Available at: https://www.cdc.gov/vaccines/covid-19/info-by-product/clinical-considera.... Accessed 4 September 2021.
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

