Neoadjuvant chemoradiotherapy followed by resection for esophageal cancer: clinical outcomes with the 'CROSS-regimen' in daily practice
- PMID: 34557905
- PMCID: PMC9016892
- DOI: 10.1093/dote/doab068
Neoadjuvant chemoradiotherapy followed by resection for esophageal cancer: clinical outcomes with the 'CROSS-regimen' in daily practice
Abstract
Background and objectives: Since the first results of the Dutch randomized CROSS-trial, neoadjuvant chemoradiotherapy (CRT) using carboplatin and paclitaxel followed by resection for primary resectable nonmetastatic esophageal cancer (EC) has been implemented as standard curative treatment in the Netherlands. The purpose of this retrospective study is to evaluate the clinical outcomes of this treatment in daily practice in a large academic hospital.
Methods: Medical records of patients treated for primary resectable nonmetastatic EC between May 2010 and December 2015 at our institution were reviewed. Treatment consisted of five weekly courses of carboplatin (area under the curve 2) and paclitaxel (50 mg/m2) with concurrent external beam radiotherapy (23 fractions of 1.8 Gy), followed by transthoracic or transhiatal resection. Data on survival, progression, acute and late toxicity were recorded.
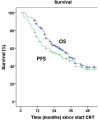
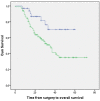
Results: A total of 145 patients were included. Median follow-up was 43 months. Median overall survival (OS) and progression-free survival (PFS) were 35 (95% confidence interval [CI] 29.8-40.2) and 30 (95% CI 19.7-40.3) months, respectively, with corresponding 3-year OS and PFS of 49.6% (95% CI 40.4-58.8) and 45.6% (95% CI 36.6-54.6). Acute toxicity grade ≥3 was observed in 25.5% of patients. Late adverse events grade ≥3 were seen in 24.8%, mostly esophageal stenosis.
Conclusion: Neoadjuvant CRT followed by resection for primary resectable nonmetastatic EC in daily practice results in a 3-year OS of 49.6% (95% CI 40.4-58.8) and PFS of 45.6% (95% CI 36.6-54.6), compared with 58% (51-65%) and 51% (43-58%) within the CROSS-trial. The slightly poorer survival in our daily practice group might be due to the presence of less favorable patient and tumor characteristics in daily practice, as is to be expected in daily practice. Toxicity was comparable with that in the CROSS-trial and considered acceptable.
Keywords: adverse effects; chemoradiotherapy; esophageal neoplasms; esophagectomy; neoadjuvant therapy.
© The Author(s) 2021. Published by Oxford University Press on behalf of International Society for Diseases of the Esophagus.
Figures


References
-
- Gupta B, Kumar N. Worldwide incidence, mortality and time trends for cancer of the oesophagus. Eur J Cancer Prev 2017; 26: 107–18. - PubMed
-
- Gebski V, Burmeister B, Smithers B M et al. Survival benefits from neoadjuvant chemoradiotherapy or chemotherapy in oesophageal carcinoma: a meta-analysis. Lancet Oncol 2007; 8: 189–90. - PubMed
-
- Sjoquist K M, Burmeister B H, Smithers B M et al. Survival after neoadjuvant chemoradiotherapy for resectable oesophageal carcinoma: an update meta-analysis. Lancet Oncol 2011; 12: 615–6. - PubMed
-
- van Hagen P, Hulshof M C C M, van Lanschot J J B et al. Preoperative chemoradiotherapy for esophageal of junctional cancer. N Engl J Med 2012; 366: 2074–84. - PubMed
-
- Shapiro J, van Lanschot J J B, Hulshof M C C M et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol 2015; 16: 1090–8. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

