Trp53 ablation fails to prevent microcephaly in mouse pallium with impaired minor intron splicing
- PMID: 34557915
- PMCID: PMC8572008
- DOI: 10.1242/dev.199591
Trp53 ablation fails to prevent microcephaly in mouse pallium with impaired minor intron splicing
Abstract
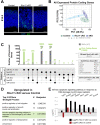
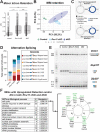
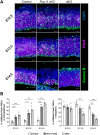
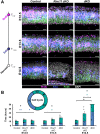
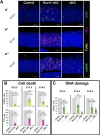
Minor spliceosome inhibition due to mutations in RNU4ATAC are linked to primary microcephaly. Ablation of Rnu11, which encodes a minor spliceosome snRNA, inhibits the minor spliceosome in the developing mouse pallium, causing microcephaly. There, cell cycle defects and p53-mediated apoptosis in response to DNA damage resulted in loss of radial glial cells (RGCs), underpinning microcephaly. Here, we ablated Trp53 to block cell death in Rnu11 cKO mice. We report that Trp53 ablation failed to prevent microcephaly in these double knockout (dKO) mice. We show that the transcriptome of the dKO pallium was more similar to the control compared with the Rnu11 cKO. We find aberrant minor intron splicing in minor intron-containing genes involved in cell cycle regulation, resulting in more severely impaired mitotic progression and cell cycle lengthening of RGCs in the dKO that was detected earlier than in the Rnu11 cKO. Furthermore, we discover a potential role of p53 in causing DNA damage in the developing pallium, as detection of γH2aX+ was delayed in the dKO. Thus, we postulate that microcephaly in minor spliceosome-related diseases is primarily caused by cell cycle defects.
Keywords: Cell cycle; Cortical development; Microcephaly; Minor spliceosome; U11 snRNA.
© 2021. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures

References
-
- Baumgartner, M., Lemoine, C., Al Seesi, S., Karunakaran, D. K. P., Sturrock, N., Banday, A. R., Kilcollins, A. M., Mandoiu, I. and Kanadia, R. N. (2015). Minor splicing snRNAs are enriched in the developing mouse CNS and are crucial for survival of differentiating retinal neurons. Dev. Neurobiol. 75, 895-907. 10.1002/dneu.22257 - DOI - PubMed
-
- Baumgartner, M., Olthof, A. M., Aquino, G. S., Hyatt, K. C., Lemoine, C., Drake, K., Sturrock, N., Nguyen, N., Al Seesi, S. and Kanadia, R. N. (2018). Minor spliceosome inactivation causes microcephaly, owing to cell cycle defects and death of self-amplifying radial glial cells. Development 145, dev166322. 10.1242/dev.166322 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

