Effects of social and emotional context on neural activation and synchrony during movie viewing
- PMID: 34558148
- PMCID: PMC8596971
- DOI: 10.1002/hbm.25669
Effects of social and emotional context on neural activation and synchrony during movie viewing
Abstract
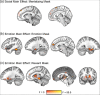
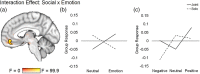
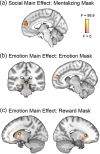
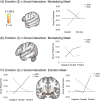
Sharing emotional experiences impacts how we perceive and interact with the world, but the neural mechanisms that support this sharing are not well characterized. In this study, participants (N = 52) watched videos in an MRI scanner in the presence of an unfamiliar peer. Videos varied in valence and social context (i.e., participants believed their partner was viewing the same (joint condition) or a different (solo condition) video). Reported togetherness increased during positive videos regardless of social condition, indicating that positive contexts may lessen the experience of being alone. Two analysis approaches were used to examine both sustained neural activity averaged over time and dynamic synchrony throughout the videos. Both approaches revealed clusters in the medial prefrontal cortex that were more responsive to the joint condition. We observed a time-averaged social-emotion interaction in the ventromedial prefrontal cortex, although this region did not demonstrate synchrony effects. Alternatively, social-emotion interactions in the amygdala and superior temporal sulcus showed greater neural synchrony in the joint compared to solo conditions during positive videos, but the opposite pattern for negative videos. These findings suggest that positive stimuli may be more salient when experienced together, suggesting a mechanism for forming social bonds.
Keywords: affect; social attention; social context; social neuroscience.
© 2021 The Authors. Human Brain Mapping published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare that they have no competing interests, financial or otherwise.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

