The oxidation-resistant CaMKII-MM281/282VV mutation does not prevent arrhythmias in CPVT1
- PMID: 34558218
- PMCID: PMC8461029
- DOI: 10.14814/phy2.15030
The oxidation-resistant CaMKII-MM281/282VV mutation does not prevent arrhythmias in CPVT1
Abstract
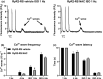
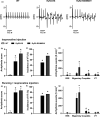
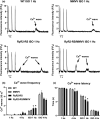
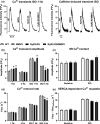
Catecholaminergic polymorphic ventricular tachycardia type 1 (CPVT1) is an inherited arrhythmogenic disorder caused by missense mutations in the cardiac ryanodine receptors (RyR2), that result in increased β-adrenoceptor stimulation-induced diastolic Ca2+ leak. We have previously shown that exercise training prevents arrhythmias in CPVT1, potentially by reducing the oxidation of Ca2+ /calmodulin-dependent protein kinase type II (CaMKII). Therefore, we tested whether an oxidation-resistant form of CaMKII protects mice carrying the CPVT1-causative mutation RyR2-R2474S (RyR2-RS) against arrhythmias. Antioxidant treatment (N-acetyl-L-cysteine) reduced the frequency of β-adrenoceptor stimulation-induced arrhythmogenic Ca2+ waves in isolated cardiomyocytes from RyR2-RS mice. To test whether the prevention of CaMKII oxidation exerts an antiarrhythmic effect, mice expressing the oxidation-resistant CaMKII-MM281/282VV variant (MMVV) were crossed with RyR2-RS mice to create a double transgenic model (RyR2-RS/MMVV). Wild-type mice served as controls. Telemetric ECG surveillance revealed an increased incidence of ventricular tachycardia and an increased arrhythmia score in both RyR2-RS and RyR2-RS/MMVV compared to wild-type mice, both following a β-adrenoceptor challenge (isoprenaline i.p.), and following treadmill exercise combined with a β-adrenoceptor challenge. There were no differences in the incidence of arrhythmias between RyR2-RS and RyR2-RS/MMVV mice. Furthermore, no differences were observed in β-adrenoceptor stimulation-induced Ca2+ waves in RyR2-RS/MMVV compared to RyR2-RS. In conclusion, antioxidant treatment reduces β-adrenoceptor stimulation-induced Ca2+ waves in RyR2-RS cardiomyocytes. However, oxidation-resistant CaMKII-MM281/282VV does not protect RyR2-RS mice from β-adrenoceptor stimulation-induced Ca2+ waves or arrhythmias. Hence, alternative oxidation-sensitive targets need to be considered to explain the beneficial effect of antioxidant treatment on Ca2+ waves in cardiomyocytes from RyR2-RS mice.
Keywords: CPVT; CaMKII; RyR2; arrhythmias; oxidation.
© 2021 The Authors. Physiological Reports published by Wiley Periodicals LLC on behalf of The Physiological Society and the American Physiological Society.
Conflict of interest statement
None declared.
Figures
References
-
- Baltogiannis, G. G., Lysitsas, D. N., di Giovanni, G., Ciconte, G., Sieira, J., Conte, G., Kolettis, T. M., Chierchia, G. B., de Asmundis, C., & Brugada, P. (2019). CPVT: arrhythmogenesis, therapeutic management, and future perspectives. A brief review of the literature. Front Cardiovascular Medicine, 6, 92. - PMC - PubMed
-
- Bezzerides, V. J., Caballero, A., Wang, S., Ai, Y., Hylind, R. J., Lu, F., Heims‐Waldron, D. A., Chambers, K. D., Zhang, D., Abrams, D. J., & Pu, W. T. (2019). Gene therapy for catecholaminergic polymorphic ventricular tachycardia by inhibition of Ca(2+)/calmodulin‐dependent kinase II. Circulation, 140, 405–419. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

