Thromboprophylaxis for Children Post-Fontan Procedure: Insights From the UNIVERSE Study
- PMID: 34558312
- PMCID: PMC8751951
- DOI: 10.1161/JAHA.120.021765
Thromboprophylaxis for Children Post-Fontan Procedure: Insights From the UNIVERSE Study
Erratum in
-
Correction to: Thromboprophylaxis for Children Post-Fontan Procedure: Insights From the UNIVERSE Study.J Am Heart Assoc. 2021 Dec 21;10(24):e020766. doi: 10.1161/JAHA.120.020766. Epub 2021 Nov 24. J Am Heart Assoc. 2021. PMID: 34816731 Free PMC article. No abstract available.
Abstract
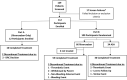
Background Patients with single-ventricle physiology who undergo the Fontan procedure are at risk for thrombotic events associated with significant morbidity and mortality. The UNIVERSE Study evaluated the efficacy and safety of a novel liquid rivaroxaban formulation, using a body weight-adjusted dosing regimen, versus acetylsalicylic acid (ASA) in children post-Fontan. Methods and Results The UNIVERSE Study was a randomized, multicenter, 2-part, open-label study of rivaroxaban, in children who had undergone a Fontan procedure, to evaluate its dosing regimen, safety, and efficacy. Part A was the single-arm part of the study that determined the pharmacokinetics/pharmacodynamics and safety of rivaroxaban in 12 participants before proceeding to part B, whereby 100 participants were randomized 2:1 to open-label rivaroxaban versus ASA. The study period was 12 months. A total of 112 participants were enrolled across 35 sites in 10 countries. In part B, for safety outcomes, major bleeding occurred in one participant on rivaroxaban (epistaxis that required transfusion). Clinically relevant nonmajor bleeding occurred in 6% of participants on rivaroxaban versus 9% on ASA. Trivial bleeding occurred in 33% of participants on rivaroxaban versus 35% on ASA. For efficacy outcomes, 1 participant on rivaroxaban in part B had a pulmonary embolism (2% overall event rate); and for ASA, 1 participant had ischemic stroke and 2 had venous thrombosis (9% overall event rate). Conclusions In this study, participants who received rivaroxaban for thromboprophylaxis had a similar safety profile and fewer thrombotic events, albeit not statistically significant, compared with those in the ASA group. Registration URL: https://www.clinicaltrials.gov. Identifier: NCT02846532.
Keywords: Fontan; children; major bleeding; rivaroxaban; thrombotic events.
Conflict of interest statement
Dr McCrindle has served on the scientific advisory board for Janssen R&D, LLC, Chiesi, and Esperion; was the Data and Safety Monitoring Board Chair for Amryt Pharma; and was an investigator for Janssen R&D, LLC. Dr Michelson has served on the scientific advisory board for Janssen R&D, LLC, AstraZeneca, Chiesi, and Stasys. Dr Justino has served on the scientific advisory board for Janssen R&D, LLC, and Pediastent; has been a consultant for Abiomed, Edwards Lifesciences, and Medtronic; has intellectual property for PolyVascular; and has a grant from W.L. Gore. Dr Harris received consultant/honoraria fees from Janssen R&D, LLC. Dr Pina is an employee with equity ownership of Janssen R&D, LLC. C. Peluso is an employee with equity ownership of Janssen R&D, LLC. K. Nessel is an employee with equity ownership of Janssen R&D, LLC. W. Lu is an employee with equity ownership of Janssen R&D, LLC. Dr Li has served on the scientific advisory board for Janssen R&D, LLC. The remaining authors have no disclosures to report.
Figures

References
-
- Monagle P, Cochrane AB, McCrindle B, Benson L, Williams W, Andrew M. Thromboembolic complications after Fontan procedures ‐ ‐ the role of prophylactic anticoagulation. J Thorac Cardiovasc Surg. 1998;115:493–498. - PubMed
-
- McCrindle BW, Manlhiot C, Cochrane A, Roberts R, Hughes M, Szechtman B, Weintraub R, Andrew M, Monagle P; Fontan Anticoagulation Study Group. Factors associated with thrombotic complications after the Fontan procedure: a secondary analysis of a multicenter, randomized trial of primary thromboprophylaxis for 2 years after the Fontan procedure. J Am Coll Cardiol. 2013;61:346–353. DOI: 10.1016/j.jacc.2012.08.1023. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

