Bile reflux and hypopharyngeal cancer (Review)
- PMID: 34558652
- PMCID: PMC8485019
- DOI: 10.3892/or.2021.8195
Bile reflux and hypopharyngeal cancer (Review)
Abstract
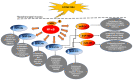
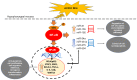
Laryngopharyngeal reflux, a variant of gastroesophageal reflux disease, has been considered a risk factor in the development of hypopharyngeal cancer. Bile acids are frequently present in the gastroesophageal refluxate and their effect has been associated with inflammatory and neoplastic changes in the upper aerodigestive tract. Recent in vitro and in vivo studies have provided direct evidence of the role of acidic bile refluxate in hypopharyngeal carcinogenesis and documented the crucial role of NF‑κB as a key mediator of early oncogenic molecular events in this process and also suggested a contribution of STAT3. Acidic bile can cause premalignant changes and invasive squamous cell cancer in the affected hypopharynx accompanied by DNA damage, elevated p53 expression and oncogenic mRNA and microRNA alterations, previously linked to head and neck cancer. Weakly acidic bile can also increase the risk for hypopharyngeal carcinogenesis by inducing DNA damage, exerting anti‑apoptotic effects and causing precancerous lesions. The most important findings that strongly support bile reflux as an independent risk factor for hypopharyngeal cancer are presented in the current review and the underlying mechanisms are provided.
Keywords: DNA damage; NF‑κB; bile reflux; conjugated bile acids; deoxycholic acid; head and neck cancer; hypopharyngeal cancer; hypopharyngeal squamous cell carcinoma; in vivo; laryngopharyngeal reflux.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Hashibe M, Boffetta P, Zaridze D, Shangina O, Szeszeni-Dabrowska N, Mates D, Fabiánová E, Rudnai P, Brennan P. Contribution of tobacco and alcohol to the high rates of squamous cell carcinoma of the supraglottis and glottis in Central Europe. Am J Epidemiol. 2007;165:814–820. doi: 10.1093/aje/kwk066. - DOI - PubMed
-
- Galli J, Cammarota G, De Corso E, Agostino S, Cianci R, Almadori G, Paludetti G. Biliary laryngopharyngeal reflux: A new pathological entity. Curr Opin Otolaryngol Head Neck Surg. 2006;14:128–132. doi: 10.1097/01.moo.0000193198.40096.be. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

