The role of pollination in controlling Ginkgo biloba ovule development
- PMID: 34558676
- PMCID: PMC9292720
- DOI: 10.1111/nph.17753
The role of pollination in controlling Ginkgo biloba ovule development
Abstract
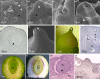
Generally, in gymnosperms, pollination and fertilization events are temporally separated and the developmental processes leading the switch from ovule integument into seed coat are still unknown. The single ovule integument of Ginkgo biloba acquires the typical characteristics of the seed coat long before the fertilization event. In this study, we investigated whether pollination triggers the transformation of the ovule integument into the seed coat. Transcriptomics and metabolomics analyses performed on ovules just prior and after pollination lead to the identification of changes occurring in Ginkgo ovules during this specific time. A morphological atlas describing the developmental stages of ovule development is presented. The metabolic pathways involved in the lignin biosynthesis and in the production of fatty acids are activated upon pollination, suggesting that the ovule integument starts its differentiation into a seed coat before the fertilization. Omics analyses allowed an accurate description of the main changes that occur in Ginkgo ovules during the pollination time frame, suggesting the crucial role of the pollen arrival on the progression of ovule development.
Keywords: Ginkgo biloba; gymnosperms; metabolomics; ovule development; ovule morphology; pollination; transcriptomics.
© 2021 The Authors. New Phytologist © 2021 New Phytologist Foundation.
Figures



References
-
- Avanzi S, Cionini PG. 1971. A DNA cytophotometric investigation on the development of the female gametophyte of Ginkgo biloba . Caryologia 24: 105–116.
-
- Barro‐Trastoy D, Dolores Gomez M, Tornero P, Perez‐Amador MA. 2020. On the way to ovules: the hormonal regulation of ovule development. Critical Reviews in Plant Sciences 39: 431–456.
-
- Becker A, Winter KU, Meyer B, Saedler H, Theißen G. 2000. MADS‐box gene diversity in seed plants 300 million years ago. Molecular Biology and Evolution 17: 1425–1434. - PubMed

