Role of Cholesterol-Associated Steatohepatitis in the Development of NASH
- PMID: 34558856
- PMCID: PMC8710790
- DOI: 10.1002/hep4.1801
Role of Cholesterol-Associated Steatohepatitis in the Development of NASH
Abstract
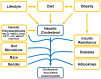
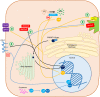
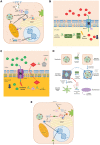
The rising prevalence of nonalcoholic fatty liver disease (NAFLD) and NAFLD-related cirrhosis in the United States and globally highlights the need to better understand the mechanisms causing progression of hepatic steatosis to fibrosing steatohepatitis and cirrhosis in a small proportion of patients with NAFLD. Accumulating evidence suggests that lipotoxicity mediated by hepatic free cholesterol (FC) overload is a mechanistic driver for necroinflammation and fibrosis, characteristic of nonalcoholic steatohepatitis (NASH), in many animal models and also in some patients with NASH. Diet, lifestyle, obesity, key genetic polymorphisms, and hyperinsulinemia secondary to insulin resistance are pivotal drivers leading to aberrant cholesterol signaling, which leads to accumulation of FC within hepatocytes. FC overload in hepatocytes can lead to ER stress, mitochondrial dysfunction, development of toxic oxysterols, and cholesterol crystallization in lipid droplets, which in turn lead to hepatocyte apoptosis, necrosis, or pyroptosis. Activation of Kupffer cells and hepatic stellate cells by hepatocyte signaling and cholesterol loading contributes to this inflammation and leads to hepatic fibrosis. Cholesterol accumulation in hepatocytes can be readily prevented or reversed by statins. Observational studies suggest that use of statins in NASH not only decreases the substantially increased cardiovascular risk, but may ameliorate liver pathology. Conclusion: Hepatic FC loading may result in cholesterol-associated steatohepatitis and play an important role in the development and progression of NASH. Statins appear to provide significant benefit in preventing progression to NASH and NASH-cirrhosis. Randomized controlled trials are needed to demonstrate whether statins or statin/ezetimibe combination can effectively reverse steatohepatitis and liver fibrosis in patients with NASH.
© 2021 The Authors. Hepatology Communications published by Wiley Periodicals LLC on behalf of American Association for the Study of Liver Diseases. This article has been contributed to by US Government employees and their work is in the public domain in the USA.
Figures

References
-
- Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018;67:328‐357. - PubMed
-
- Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol 2018;15:11‐20. - PubMed
-
- Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease—meta‐analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64:73‐84. - PubMed
-
- Beste LA, Leipertz SL, Green PK, Dominitz JA, Ross D, Ioannou GN. Trends in burden of cirrhosis and hepatocellular carcinoma by underlying liver disease in US veterans, 2001‐2013. Gastroenterology 2015;149:1471‐1482.e5; quiz e17‐8. - PubMed
-
- Wong RJ, Aguilar M, Cheung R, Perumpail RB, Harrison SA, Younossi ZM, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology 2015;148:547‐555. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

