Comparative efficacy and safety for second-line treatment with ramucirumab, regorafenib, and cabozantinib in patients with advanced hepatocellular carcinoma progressed on sorafenib treatment: A network meta-analysis
- PMID: 34559096
- PMCID: PMC8462645
- DOI: 10.1097/MD.0000000000027013
Comparative efficacy and safety for second-line treatment with ramucirumab, regorafenib, and cabozantinib in patients with advanced hepatocellular carcinoma progressed on sorafenib treatment: A network meta-analysis
Abstract
Background: The present network meta-analysis was conducted to perform an indirect comparison among ramucirumab, regorafenib, and cabozantinib in patients with advanced hepatocellular carcinoma (HCC) progressed on sorafenib treatment.
Methods: A systematic review through Medline, Embase, and Cochrane library was developed, with eligible randomized clinical trials been included. Hazard ratios (HRs) including progression-free survival (PFS), overall survival (OS), odds ratios of disease control rate (DCR), objective response rate (ORR), and adverse events were compared indirectly with network meta-analysis using random model in software STATA version 13.0.
Results: A total of 4 randomized clinical trials including 2137 patients met the eligibility criteria and enrolled. Indirect comparisons showed that there was no statistical difference observed in the indirect comparison of PFS, OS, ORR, or DCR among agents of regorafenib, cabozantinib, and ramucirumab in advanced HCC patients with elevated α-fetoprotein (AFP) (400 ng/mL or higher). However, in patients with low-level AFP (lower than 400 ng/mL), regorafenib was the only agent associated with significant superiority in OS, compared with placebo (hazard ratio 0.67, 95% CI, 0.50-0.90).
Conclusions: The present network meta-analysis revealed that there might be no statistical difference observed in the indirect comparison of PFS, OS, ORR, or DCR among regorafenib, cabozantinib, or ramucirumab in advanced HCC patients with elevated AFP (400 ng/mL or higher). However, in patients with low-level AFP (lower than 400 ng/mL), regorafenib might be associated with significant superiority in OS, compared to placebo, which need further investigation in clinical practice.
Copyright © 2021 the Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
The authors have no funding and conflicts of interest to disclose.
Figures






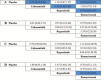
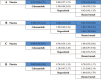
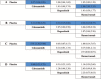
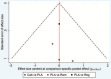
References
-
- Akinyemiju T, Abera S, Ahmed M, et al. . C. Global Burden of Disease Liver Cancer. The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level: results from the global burden of disease study 2015. JAMA Oncol 2017;3:1683–91. - PMC - PubMed
-
- Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology 2004;127: (Suppl 1): S35–50. - PubMed
-
- European Association For The Study Of The Liver, European Organisation For Research And Treatment of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012;56:908–43. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

